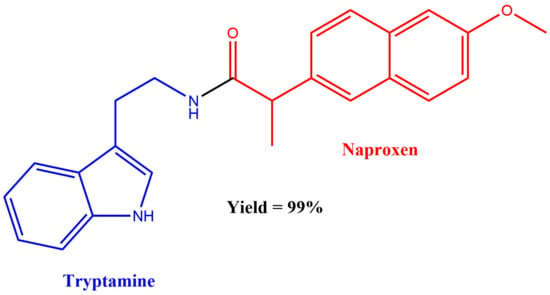
N-(2-(1H-Indol-3-yl)ethyl)-2-(6-methoxynaphthalen-2-yl)propanamide
Abstract
:1. Introduction
2. Results
3. Materials and Methods
Synthesis of N-(2-(1H-indol-3-yl)ethyl)-2-(6-methoxynaphthalen-2-yl)propanamide 4
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brutzus, J.; Varacallo, M.; Varacallo, M. Naproxen, NCBI Bookshelf, StatPearls Publishing 2020 Bookshelf ID: NBK525965PMID: 30247840. Available online: https://www.ncbi.nlm.nih.gov/books/NBK525965/?report=printable (accessed on 13 January 2020).
- Hung, I.; To, K.; Chan, J.; Cheng, V.; Liu, K.; Tam, A.; Chan, T.; Zhang, A.; Li, P.; Wong, T.; et al. Efficacy of Clarithromycin-Naproxen-Oseltamivir combination in the treatment of patients hospitalized for Influenza A(H3N2) infection: An Open-label Randomized, Controlled, Phase IIb/III Trial. Chest 2017, 151, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Adnet, F.; Schwo, A.S. Efficacy of Addition of Naproxen in the Treatment of Critically Ill Patients Hospitalized for COVID-19 Infection (ENACOVID). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04325633 (accessed on 13 January 2020).
- Dexter, D.; Hesson, D.; Ardecky, R.; Rao, G.; Tippett, D.; Dusak, B.; Paull, K.; Plowman, J.; DeLarco, B.; Narayanan, V.; et al. Activity of a novel 4-quinolinecarboxylic acid, NSC 368390 [6-fluoro-2-(2’-fluoro-1,1’-biphenyl-4-yl)-3-methyl-4-quinolinecarboxylic acid sodium salt], against experimental tumors. Cancer Res. 1985, 45, 5563–5568. [Google Scholar] [PubMed]
- Jenkins, T.; Nguen, J.; Polglaze, K.; Bertrand, P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Tylš, F.; Páleníček, T.; Horáček, J. Psilocibin—Summary of knowledge and new perspectives. Eur. Neuropsychopharmacol. 2014, 24, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Tittarelli, R.; Mannocchi, G.; Pantano, F.; Romolo, F. Recreational use, analysis and toxicity of tryptamines. Curr. Neuropharmacol. 2015, 13, 26–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, S.; Freeman, S.; McGagh, P.; Abdul-Halim, N.; Alder, J. An analytical perspective on favored synthetic routes to the psychoactive tryptamines. J. Pharm. Biomed. Anal. 2004, 36, 375–691. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Freeman, S.; Alder, J.; Passie, T.; Brandt, S. Profiling psychoactive tryptamine-drug synthesis by focusing on detection using mass spectroscopy. Trends Analyt. Chem. 2010, 29, 285–296. [Google Scholar] [CrossRef]
- Rose, T.M.; Reilly, C.A.; Deering-Rice, C.E.; Brewster, C.; Brewster, C. Inhibition of FAAH, TRPV1, and COX2 by NSAID-serotonin conjugates. Bioorg. Med. Chem. Lett. 2014, 24, 5695–5698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trabocchi, A.; Mannino, C.; Machetti, F.; Bernardis, D.F.; Arancia, S.; Cauda, R.; Cassone, A.; Guarna, A. Identification of inhibitors of drug-resistant Candida albicans strains from a library of bicyclic peptidomimetic compounds. J. Med. Chem. 2010, 53, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Rees, D.; Rankovic, Z.; Morphy, R. Synthesis of tertiary amines using a polystyrene (REM) resin. J. Am. Chem. Soc. 1997, 119, 3288–3295. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolov, S.; Ivanov, I.; Bojilov, D. N-(2-(1H-Indol-3-yl)ethyl)-2-(6-methoxynaphthalen-2-yl)propanamide. Molbank 2021, 2021, M1187. https://doi.org/10.3390/M1187
Manolov S, Ivanov I, Bojilov D. N-(2-(1H-Indol-3-yl)ethyl)-2-(6-methoxynaphthalen-2-yl)propanamide. Molbank. 2021; 2021(1):M1187. https://doi.org/10.3390/M1187
Chicago/Turabian StyleManolov, Stanimir, Iliyan Ivanov, and Dimitar Bojilov. 2021. "N-(2-(1H-Indol-3-yl)ethyl)-2-(6-methoxynaphthalen-2-yl)propanamide" Molbank 2021, no. 1: M1187. https://doi.org/10.3390/M1187
APA StyleManolov, S., Ivanov, I., & Bojilov, D. (2021). N-(2-(1H-Indol-3-yl)ethyl)-2-(6-methoxynaphthalen-2-yl)propanamide. Molbank, 2021(1), M1187. https://doi.org/10.3390/M1187