Ammonium Salts of 5-(3-Chromenyl)-5H-chromeno[2,3-b]pyridines
Abstract
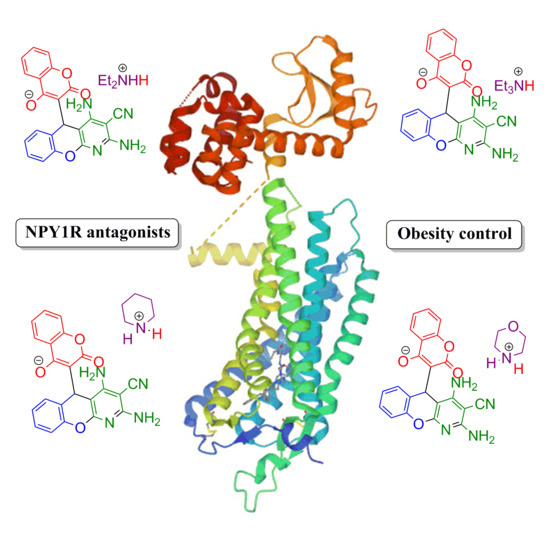
:1. Introduction
2. Results and Discussion
2.1. Multicomponent Synthesis of Ammonium Salts of 5-(3-Chromenyl)-5H-chromeno[2,3-b]pyridines 5
2.2. NMR Spectroscopy Study of Morpholin-4-ium 3-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)-2-oxo-2H-chromen-4-olate 5d
2.3. Acidification of Ammonium Salts of 2,4-Diamino-5-(4-Hydroxy-2-oxo-2H-chromen-3-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 5
3. Materials and Methods
3.1. General Methods
3.2. Multicomponent Synthesis of Ammonium Salts of 2,4-Diamino-5-(4-Hydroxy-2-oxo-2H-chromen-3-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 5
3.3. Acidification of Ammonium Salts of 2,4-Diamino-5-(4-Hydroxy-2-oxo-2H-chromen-3-yl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.; Soni, M.; Kumar, S.; Gupta, G.D. Solubility enhancement—eminent role in poorly soluble drugs. Res. J. Pharm. Technol. 2009, 2, 220–224. [Google Scholar]
- Patel, M.S.N.; Ahmed, M.H.; Saqib, M.; Shaikh, S.N. Chemical Modification: A unique solutions to Solubility problem. J. Drug Deliv. Ther. 2019, 9, 542–546. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.K.; Tripathi, R.P.; Ramachandran, R. NAD+-dependent DNA Ligase (Rv3014c) from Mycobacterium tuberculosis. J. Biol. Chem. 2005, 280, 30273–30281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuine, M. Cancer chemopreventive effect of phenothiazines and related tri-heterocyclic analogues in the 12-O-tetradecanoylphorbol-13-acetate promoted Epstein-Barr virus early antigen activation and the mouse skin two-stage carcinogenesis models. Pharmacol. Res. 2004, 49, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Goto, K.; Terasawa, M.; Nakatsu, O. Use of 9-chloro-7-(1H-tetrazol-5-yl)-5-oxo-5H[1H]benzopyrano[2,3-b]-pyridine and its Salts and Hydrates in the Treatment of Rheumatic. Maladies. Patent DE 3010751, 17 January 1980. [Google Scholar]
- Shigemitsu, T.; Watanabe, N. Composition for Prophylaxis and Treatment of. Myopia. Patent EP0647445A1, 5 October 1994. [Google Scholar]
- Ukawa, K.; Ishiguro, T.; Kuriki, H.; Nohara, A. Synthesis of the metabolites and degradation products of 2-amino-7-isopropyl-5-oxo-5H-(1)benzopyrano(2,3-b)pyridine-3-carboxylic acid (Amoxanox). Chem. Pharm. Bull. 1985, 33, 4432–4437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elinson, M.N.; Ryzhkova, Y.E.; Ryzhkov, F.V. Multicomponent design of chromeno[2,3-b]pyridine systems. Russ. Chem. Rev. 2021, 90, 94–115. [Google Scholar] [CrossRef]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Ryzhkov, F.V.; Ryzhkova, Y.E.; Elinson, M.N.; Vereshchagin, A.N.; Korolev, V.A.; Egorov, M.P. Quadruple Bond Forming Multicomponent Approach to 5-(3-chromenyl)-5H-chromeno[2,3-b]pyridines and Its Interaction with the Neuropeptide Y1 Receptor. Chem. Heterocycl. Compd. 2020, 56, 1560–1568. [Google Scholar] [CrossRef]
- Ryzhkov, F.V.; Ryzhkova, Y.E.; Elinson, M.N.; Vorobyev, S.V.; Fakhrutdinov, A.N.; Vereshchagin, A.N.; Egorov, M.P. Catalyst-Solvent System for PASE Approach to Hydroxyquinolinone-Substituted Chromeno[2,3-b]pyridines Its Quantum Chemical Study and Investigation of Reaction Mechanism. Molecules 2020, 25, 2573. [Google Scholar] [CrossRef] [PubMed]
- Patai, S.; Israeli, Y. 411. The kinetics and mechanisms of carbonyl–methylene condensations. Part VII. The reaction of malononitrile with aromatic aldehydes in ethanol. J. Chem. Soc. 1960, 2025–2030. [Google Scholar] [CrossRef]
- Stanley, B.G.; Kyrkouli, S.E.; Lampert, S.; Leibowitz, S.F. Neuropeptide Y chronically injected into the hypothalamus: A powerful neurochemical inducer of hyperphagia and obesity. Peptides 1986, 7, 1189–1192. [Google Scholar] [CrossRef]
- Behr, A.; Becker, M.; Reyer, S. A highly efficient method for the hydroaminomethylation of long-chain alkenes under aqueous, biphasic conditions. Tetrahedron Lett. 2010, 51, 2438–2441. [Google Scholar] [CrossRef]
- Mittelbach, M. An improved and facile synthesis of 2-amino-1,1,3-tricyanopropene. Mon. Chem. Chem. Mon. 1985, 116, 689–691. [Google Scholar] [CrossRef]





 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkova, Y.E.; Fakhrutdinov, A.N.; Elinson, M.N. Ammonium Salts of 5-(3-Chromenyl)-5H-chromeno[2,3-b]pyridines. Molbank 2021, 2021, M1219. https://doi.org/10.3390/M1219
Ryzhkova YE, Fakhrutdinov AN, Elinson MN. Ammonium Salts of 5-(3-Chromenyl)-5H-chromeno[2,3-b]pyridines. Molbank. 2021; 2021(2):M1219. https://doi.org/10.3390/M1219
Chicago/Turabian StyleRyzhkova, Yuliya E., Artem N. Fakhrutdinov, and Michail N. Elinson. 2021. "Ammonium Salts of 5-(3-Chromenyl)-5H-chromeno[2,3-b]pyridines" Molbank 2021, no. 2: M1219. https://doi.org/10.3390/M1219
APA StyleRyzhkova, Y. E., Fakhrutdinov, A. N., & Elinson, M. N. (2021). Ammonium Salts of 5-(3-Chromenyl)-5H-chromeno[2,3-b]pyridines. Molbank, 2021(2), M1219. https://doi.org/10.3390/M1219