Synthesis and Cytotoxic Potential of 3-Oxo-19β-trifluoroacetoxy-18αH-oleane-28-oic Acid
Abstract
:1. Introduction
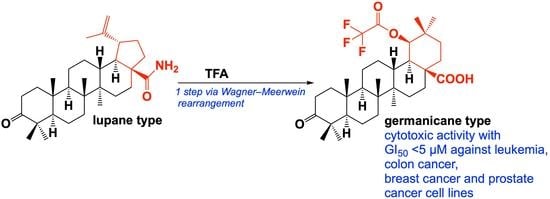
2. Results and Discussion
3. Materials and Methods
Synthesis of Compounds 2 and 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ren, Y.; Kinghorn, A.D. Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Med. 2019, 85, 802–814. [Google Scholar] [CrossRef] [Green Version]
- Sporn, M.B.; Liby, K.; Yore, M.M.; Suh, N.; Albini, A.; Honda, T.; Sundararajan, C.; Gribble, G.W. Platforms and networks in triterpenoid pharmacology. Drug Dev. Res. 2007, 68, 174–182. [Google Scholar] [CrossRef]
- Dehaen, W.; Mashentseva, A.A.; Seitembetov, T.S. Allobetulin and its derivatives: Synthesis and biological activity. Molecules 2011, 16, 2443–2466. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Medvedeva, N.I.; Kazakov, D.V.; Tolstikov, G.A. A Method of Producing Allobetulin. RU 2402561C1, 27 October 2010. [Google Scholar]
- Khusnutdinova, E.F.; Petrova, A.V.; Ha Nguyen, T.T.; Anh Le, T.T.; Tra Nguyen, T.; Cham, B.T.; Babkov, D.A.; Kazakova, O.B. Structural modifications of 2,3-indolobetulinic acid: Design and synthesis of highly potent α-glucosidase inhibitors. Bioorg. Chem. 2019, 88, 102957. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Smirnova, I.E.; Kazakova, O.B. Synthesis and cytotoxicity of 28-oxo-allobetulone derivatives. Chem. Nat. Compd. 2020, 56, 465–471. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Kazakova, O.B.; Lobov, A.N.; Kukovinets, O.S.; Suponitsky, K.Y.; Meyers, C.B.; Prichard, M.N. Synthesis of A-ring quinolones, nine-membered oxolactams and spiroindoles by oxidative transformations of 2,3-indolotriterpenoids. Org. Biomol. Chem. 2019, 17, 585–597. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Smirnova, I.E.; Khusnutdinova, E.F.; Zhukova, O.S.; Fetisova, L.V.; Apryshko, G.N.; Medvedeva, N.I.; Yamansarov, E.Y.; Baikova, I.P.; Nguyen, T.T.; et al. Synthesis and cytotoxicity of allobetulin derivatives. Russ. J. Bioorg. Chem. 2014, 40, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.F.; Medvedeva, N.I.; Kazakov, D.V.; Kukovinets, O.S.; Lobov, A.N.; Suponitsky, K.Y.; Kazakova, O.B. An efficient synthesis of moronic and heterobetulonic acids from allobetulin. Tetrahedron Lett. 2016, 57, 148–151. [Google Scholar] [CrossRef]
- Babaev, M.; Khusnutdinova, E.; Lobov, A.; Galimova, Z.; Petrova, A.; Rybalova, T.; Ha Nguen, T.T.; Meyers, C.; Prichard, M.; Kazakova, O. Allobetulone rearrangement to l8αH,19βH-ursane triterpenoids with antiviral activity. Nat. Prod. Res. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Nazarov, M.A.; Tolmacheva, I.A.; Grishko, V.V. The synthesis of α,β-unsaturated 18αH,19βH-ursane methyl ketones. Arkivok 2019, 6, 267–276. [Google Scholar] [CrossRef]
- Dorr, C.R.; Yemets, S.; Kolomitsyna, O.; Krasutsky, P.; Mansky, L.M. Triterpene derivatives that inhibit human immunodeficiency virus type 1 replication. Bioorg. Med. Chem. Lett. 2011, 21, 542–545. [Google Scholar] [CrossRef] [Green Version]
- Schulze, H.; Pieroh, K. Zur Kenntnis des Betulins. Ber. Dtsch. Chem. Ges. 1922, 55, 2332–2346. [Google Scholar] [CrossRef] [Green Version]
- Pakulski, Z.; Cmoch, P.; Korda, A.; Luboradzki, R.; Gwardiak, K.; Karczewski, R. Rearrangements of the betulin core. Synthesis of terpenoids possessing the bicyclo[3.3.1]nonane fragment by rearrangement of lupane-type epoxides. J. Org. Chem. 2021, 86, 1084–1095. [Google Scholar] [CrossRef]
- Csuk, R. Betulinic acid and its derivatives: A patent review (2008–2013). Expert Opin. Ther. Pat. 2014, 24, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A. Betulin and betulinic acid in cancer research. J. Pre-Clin. Clin. Res. 2018, 12, 72–75. [Google Scholar] [CrossRef]
- Drąg-ZalesiĔska, M.; Borska, S. Betulin and its derivatives–precursors of new drugs. World Sci. News 2019, 127, 123–138. [Google Scholar]
- Kommera, H.; Kaluderovic, G.N.; Kalbitz, J.; Drager, B.; Paschke, R. Small structural changes of pentacyclic lupane type triterpenoid derivatives lead to significant differences in their anticancer properties. Eur. J. Med. Chem. 2010, 45, 3346–3353. [Google Scholar] [CrossRef]
- Lombrea, A.; Scurtu, A.D.; Avram, S.; Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Dehelean, C.A.; Soica, C.; Danciu, C. Anticancer potential of betulonic acid derivatives. Int. J. Mol. Sci. 2021, 22, 3676. [Google Scholar] [CrossRef] [PubMed]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. Cancer Institute: Cancer drug discovery and development program. Semin. Oncol. 1992, 19, 622–638. [Google Scholar]
- Weinstein, J.N.; Myers, T.G.; O’Connor, P.M.; Friend, S.H.; Fornace, A.J.; Kohn, K.W.; Fojo, T.; Bates, S.E.; Rubinstein, L.V.; Anderson, N.L.; et al. An information-intensive approach to the molecular pharmacology of cancer. Science 1997, 275, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Kazakova, O.B.; Giniyatullina, G.V.; Mustafin, A.G.; Babkov, D.A.; Sokolova, E.V.; Spasov, A.A. Evaluation of cytotoxicity and α-glucosidase inhibitory activity of amide and polyamino-derivatives of lupane triterpenoids. Molecules 2020, 25, 4833. [Google Scholar] [CrossRef]
- DTP Databasesand Search Tools. 2018. Available online: https://dtp.cancer.gov/databases_tools/data_search.htm (accessed on 1 November 2018).



| Compound | 60 Cell Lines Assay in 1-Dose at 10 µM | ||
|---|---|---|---|
| Mean Growth, % | Range of Growth, % | Most Sensitive Cell Line | |
| 2 | 77.29 | 10.97 to 119.95 | HOP-92 (Non-Small Cell Lung Cancer) |
| 3 | −62.67 | −99.13 to 1.66 | HL-60(TB) (Leukemia) |
| Subpanel/Cell Lines (μM) | GI50 | Subpanel/Cell Lines (μM) | GI50 |
|---|---|---|---|
| Leukemia | Melanoma | ||
| CCRF-CEM | 9.51 | LOX IMVI | 5.91 |
| HL-60(TB) | 3.16 | MALME-3M | 12.90 |
| K-562 | 3.61 | M14 | 8.34 |
| MOLT-4 | 4.22 | MDA-B-435 | 10.60 |
| RPMI-8226 | 4.82 | SK-MEL-2 | 14.10 |
| SR | 3.75 | SK-MEL-28 | 13.60 |
| Non-Small Cell Lung Cancer | SK-MEL-5 | 8.11 | |
| A549/ATCC | 9.66 | UACC-257 | 10.10 |
| EKVX | 14.50 | UACC-62 | 6.67 |
| HOP-62 | 13.20 | Ovarian Cancer | |
| HOP-92 | 8.08 | IGROV1 | 18.90 |
| NCI-H226 | 13.50 | OVCAR-3 | 12.40 |
| NCI-H23 | 11.00 | OVCAR-4 | 13.10 |
| NCI-H322M | 17.80 | OVCAR-5 | 15.70 |
| NCI-H460 | 7.65 | OVCAR-8 | 12.90 |
| NCI-H522 | 12.60 | NCI/ADR-RES | 12.30 |
| Colon cancer | SK-OV-3 | 13.40 | |
| COLO 205 | 6.68 | Renal Cancer | |
| HCC-2998 | 10.70 | 786-0 | 14.30 |
| HCT-116 | 4.80 | A498 | 15.70 |
| HCT-15 | 4.99 | ACHN | 13.30 |
| HT29 | 4.06 | CAKI-1 | 12.80 |
| KM-12 | 10.40 | RXF 393 | 11.10 |
| SW-620 | 11.60 | SN12C | 11.50 |
| CNS Cancer | TK-10 | 16.90 | |
| SF-268 | 15.30 | UO-31 | 13.70 |
| SF-295 | 14.60 | Breast Cancer | |
| SF-539 | 5.34 | MCF7 | 5.02 |
| SNB-19 | 13.80 | MDA-MB-231/ATCC | 13.50 |
| SNB-75 | 26.40 | HS 578T | 20.30 |
| U251 | 12.40 | BT-549 | 9.30 |
| Prostate Cancer | T -47D | 3.84 | |
| PC-3 | 3.98 | MDA-MB-468 | 5.83 |
| DU-145 | 14.10 | ||
| Compound | Gram–Positive Bacteria | Gram–Negative Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|---|
| Staphylococcus Aureus | Escherichia coli | Klebsiella pneumonia | Pseudomonas aeruginosa | Acinetobacter baumannii | Candida albicans | Cryptococcus neoformans var. grubii | |
| Strain ATCC43300 | Strain ATCC 25922 | Strain ATCC 700603 | Strain 19606 | Strain ATCC 27853 | Strain ATCC 90028 | Strain H99, ATCC 208821 | |
| 2 | 10.58 | −6.72 | 10.6 | 4.47 | 16.9 | 10.93 | −8.97 |
| 3 | −4.52 | −6.07 | −2.26 | 10.26 | 25.36 | 6.12 | −13.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khusnutdinova, E.F.; Poptsov, A.I.; Kazakova, O.B. Synthesis and Cytotoxic Potential of 3-Oxo-19β-trifluoroacetoxy-18αH-oleane-28-oic Acid. Molbank 2021, 2021, M1222. https://doi.org/10.3390/M1222
Khusnutdinova EF, Poptsov AI, Kazakova OB. Synthesis and Cytotoxic Potential of 3-Oxo-19β-trifluoroacetoxy-18αH-oleane-28-oic Acid. Molbank. 2021; 2021(2):M1222. https://doi.org/10.3390/M1222
Chicago/Turabian StyleKhusnutdinova, Elmira F., Alexander I. Poptsov, and Oxana B. Kazakova. 2021. "Synthesis and Cytotoxic Potential of 3-Oxo-19β-trifluoroacetoxy-18αH-oleane-28-oic Acid" Molbank 2021, no. 2: M1222. https://doi.org/10.3390/M1222
APA StyleKhusnutdinova, E. F., Poptsov, A. I., & Kazakova, O. B. (2021). Synthesis and Cytotoxic Potential of 3-Oxo-19β-trifluoroacetoxy-18αH-oleane-28-oic Acid. Molbank, 2021(2), M1222. https://doi.org/10.3390/M1222