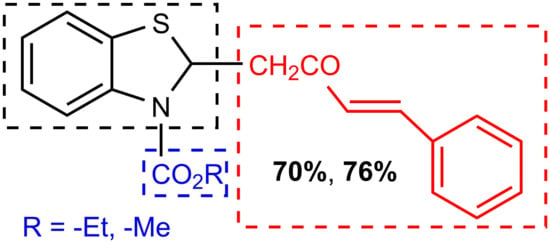
(E)-2-(2-Oxo-4-phenylbut-3-en-1-yl)benzo[d]thiazole-3(2H)-carboxylates
Abstract
:1. Introduction
2. Results
3. Materials and Methods
Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denny, W.A. The contribution of synthetic organic chemistry to anticancer drug development. Anticancer Drug Dev. 2002, 11, 187–202. [Google Scholar] [CrossRef]
- Liang, G.; Fan, W.; Luo, H.; Zhu, X. The emerging roles of artificial intelligence in cancer drug development and precision therapy. Biomed. Pharmacother. 2020, 128, 110255. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Rawal, R.K.; Bariwal, J. Recent Advances in the Chemistry and Biology of Benzothiazoles. Arch. Pharm. (Weinh.) 2015, 348, 155–178. [Google Scholar] [CrossRef] [PubMed]
- Irfan, A.; Batool, F.; Naqvi, S.A.Z.; Islam, A.; Osman, S.M.; Nocentini, A.; Alissa, S.A.; Supuran, C.T. Benzothiazole derivatives as anticancer agents. J. Enzyme Inhib. Med. Chem. 2020, 35, 265–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, J.; Khan, A.A.; Ali, Z.; Haider, R.; Yar, M.S. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem. 2017, 125, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Sharma, D.; Sharma, A.; Bansal, K.K.; Rajak, H.; Sharma, S.; Thakur, V.K. New horizons in benzothiazole scaffold for cancer therapy: Advances in bioactivity, functionality, and chemistry. Appl. Mater. Today 2020, 20, 100783. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S. Benzothiazoles: How Relevant in Cancer Drug Design Strategy? Anticancer Agents Med. Chem. 2014, 14, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Ammazzalorso, A.; Carradori, S.; Amoroso, R.; Fernández, I.F. 2-substituted benzothiazoles as antiproliferative agents: Novel insights on structure-activity relationships. Eur. J. Med. Chem. 2020, 207, 112762. [Google Scholar] [CrossRef] [PubMed]
- Uremis, N.; Uremis, M.M.; Tolun, F.I.; Ceylan, M.; Doganer, A.; Kurt, A.H. Synthesis of 2-Substituted Benzothiazole Derivatives and Their In Vitro Anticancer Effects and Antioxidant Activities Against Pancreatic Cancer Cells. Anticancer Res. 2017, 37, 6381–6389. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, E.B.; de Brito, M.A.; Vasconcelos, T.R.A.; de Moraes, M.O.; Montenegro, R.C.; Yoneda, J.D.; Leal, K.Z. Synthesis and anticancer activity of (E)-2-benzothiazole hydrazones. Eur. J. Med. Chem. 2014, 86, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Osmaniye, D.; Levent, S.; Karaduman, A.B.; Ilgın, S.; Özkay, Y.; Kaplancikli, Z.A. Synthesis of New Benzothiazole Acylhydrazones as Anticancer Agents. Molecules 2018, 23, 1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.G.; Zhao, H.; Lan, Y.B.; Wu, B.; Huang, X.F.; Chen, J.; Tao, J.C.; Wang, X.W. Asymmetric cross aldol addition of isatins with α,β-unsaturated ketones catalyzed by a bifunctional Brønsted acid–Brønsted base organocatalyst. Tetrahedron 2012, 68, 3843–3850. [Google Scholar] [CrossRef]
- Eberle, M.; Bachmann, F.; Strebel, A.; Roy, S.; Srivastava, S.; Saha, G. Furazanobenzimidazoles. Patent WO2004103994A1. Available online: https://patents.google.com/patent/WO2004103994A1 (accessed on 2 December 2004).
- Cao, B.; Wang, Y.; Ding, K.; Neamati, N.; Long, Y.Q. Synthesis of the pyridinyl analogues of dibenzylideneacetone (pyr-dba) via an improved Claisen–Schmidt condensation, displaying diverse biological activities as curcumin analogues. Org. Biomol. Chem. 2012, 10, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Reddy, V.B.; Kumar, A.; Mandal, D.; Tiwari, R.; Parang, K. Click chemistry inspired one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles and their Src kinase inhibitory activity. Bioorganic Med. Chem. Lett. 2011, 21, 449–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkov, A.P.; Statkova-Abeghe, S. α-Amidoalkylation Reactions and Oxidation of Adducts of Benzimidazoles and Acyl Chlorides. Synth. Commun. 1998, 28, 1857–1864. [Google Scholar] [CrossRef]
- Statkova-Abeghe, S.; Ivanov, I.; Daskalova, S.; Dzhambazov, B. Synthesis and Cytotoxic Evaluation of 1,2,3-Trisubstituted 2-(2-Oxoalkyl)-2,3-dihydrobenzimidazoles. Med. Chem. Res. 2005, 14, 429–439. [Google Scholar] [CrossRef]
- Stremski, Y.; Statkova-Abeghe, S.; Angelov, P.; Ivanov, I. Synthesis of Camalexin and Related Analogues. J. Heterocycl. Chem. 2018, 55, 1589–1595. [Google Scholar] [CrossRef]
- Stremski, Y.; Kirkova, D.; Statkova-Abeghe, S.; Angelov, P.; Ivanov, I.; Georgiev, D. Synthesis and antibacterial activity of hydroxylated 2-arylbenzothiazole derivatives. Synth. Commun. 2020, 50, 3007–3015. [Google Scholar] [CrossRef]


| Product 4 | R | Time, h | T, °C | Yield, % | |
|---|---|---|---|---|---|
| a | −Et | 80 | 25 | 50 | 70 * |
| b | −Me | 80 | 25 | 55 | 76 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stremski, Y.; Statkova-Abeghe, S. (E)-2-(2-Oxo-4-phenylbut-3-en-1-yl)benzo[d]thiazole-3(2H)-carboxylates. Molbank 2021, 2021, M1236. https://doi.org/10.3390/M1236
Stremski Y, Statkova-Abeghe S. (E)-2-(2-Oxo-4-phenylbut-3-en-1-yl)benzo[d]thiazole-3(2H)-carboxylates. Molbank. 2021; 2021(2):M1236. https://doi.org/10.3390/M1236
Chicago/Turabian StyleStremski, Yordan, and Stela Statkova-Abeghe. 2021. "(E)-2-(2-Oxo-4-phenylbut-3-en-1-yl)benzo[d]thiazole-3(2H)-carboxylates" Molbank 2021, no. 2: M1236. https://doi.org/10.3390/M1236
APA StyleStremski, Y., & Statkova-Abeghe, S. (2021). (E)-2-(2-Oxo-4-phenylbut-3-en-1-yl)benzo[d]thiazole-3(2H)-carboxylates. Molbank, 2021(2), M1236. https://doi.org/10.3390/M1236