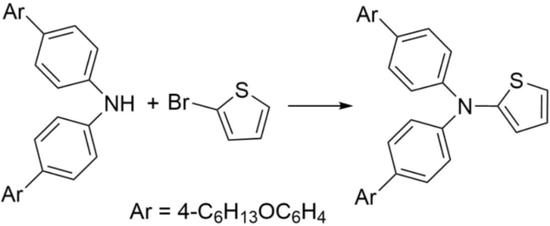
N,N-Bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)thiophen-2-amine
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yahya, M.; Bouziani, A.; Ocak, C.; Seferoğlu, Z.; Sillanpää, M. Organic/metal-organic photosensitizers for dye-sensitized solar cells (DSSC): Recent developments, new trends, and future perceptions. Dye. Pigment. 2021, 192, 109227. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Li, X.; Mao, J.; Wu, W.; Ågren, H.; Hua, J. Photovoltaic properties of bis(octyloxy)benzo-[c][1,2,5]thiadiazole sensitizers based on an N,N-diphenylthiophen-2-amine donor. J. Mater. Chem. C 2014, 2, 4063–4072. [Google Scholar] [CrossRef]
- Capodilupo, A.-L.; Fabiano, E.; Franco, L.; Gambino, S.; Leoncini, M.; Accorsi, G.; Gigli, G. Control of Electron Transfer Processes in Multidimensional Arylamine-Based Mixed-Valence Compounds by Molecular Backbone Design. J. Phys. Chem. A 2021, 125, 7840–7851. [Google Scholar] [CrossRef] [PubMed]
- Choua, S.-H.; Chen, H.-C.; Wang, C.-K.; Chung, C.-L.; Hung, C.-M.; Hsu, J.-C.; Wong, K.-T. Synthesis and characterization of new asymmetric thieno [3,4-b]pyrazinebased D−π−A−A type small molecular donors with near-infrared absorption and their photovoltaic applications. Org. Electron. 2019, 68, 159–167. [Google Scholar] [CrossRef]
- Chen, C.-H.; Luo, Z.-H.; Huan, I.-H.; Chen, Y.-H.; Lim, T.-S. Rationalize the roles of electron donating-withdrawing groups in the impacts on solvatochromism, nonlinear optics, and electroluminescence devices. Dye. Pigment. 2020, 175, 10843. [Google Scholar] [CrossRef]
- Chen, C.; Ni, X.; Jia, S.; Liang, Y.; Wu, X.; Kong, D.; Ding, D. Massively Evoking Immunogenic Cell Death by Focused Mitochondrial Oxidative Stress using an AIE Luminogen with a Twisted Molecular Structure. Adv. Mater. 2019, 31, 1904914. [Google Scholar] [CrossRef] [PubMed]
- Wild, M.; Griebel, J.; Hajduk, A.; Friedrich, D.; Stark, A.; Abel, B.; Siefermann, K.R. Efficient synthesis of triarylaminebased dyes for p-type dyesensitized solar cells. Sci. Rep. 2016, 6, 26263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Zhou, H.; Zhang, S.; Gea, C.; Cheng, S. Influence of the auxiliary acceptor and π-bridge in triarylamine dyes on dye-sensitized solar cells. Photochem. Photobiol. Sci. 2019, 18, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Chmovzh, T.N.; Rakitin, O.A. tert-Butyl Bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)carbamate. Molbank 2021, 2021, M1247. [Google Scholar] [CrossRef]
- Watanabe, M.; Yamamoto, T.; Nishiyama, M. Synthesis of novel (bis)(diarylamino)thiophenes via palladium-catalysed reaction of (di)bromothiophenes with diarylamines. Chem. Commun. 2000, 2, 133–134. [Google Scholar] [CrossRef]
- Capodilupo, A.-L.; Vergaro, V.; Accorsi, G.; Fabiano, E.; Baldassarre, F.; Corrente, G.A.; Gigli, G.; Ciccarella, G. A series of diphenylamine-fluorenone derivatives as potential fluorescent probes for neuroblastoma cell staining. Tetrahedron 2016, 72, 2920–2928. [Google Scholar] [CrossRef]
- Shimogawa, H.; Murata, Y.; Wakamiya, A. NIR-Absorbing Dye Based on BF2-Bridged Azafulvene Dimer as a Strong Electron-Accepting Unit. Org. Lett. 2018, 20, 5135–5138. [Google Scholar] [CrossRef] [PubMed]

| Entry | Solvent | Temperature, °C | Time, h | Yield, of 2% |
|---|---|---|---|---|
| 1 | Benzene | 78 | 24 | 10 |
| 2 | Toluene | 110 | 24 | 30 |
| 3 | Xylene | 120 | 24 | 45 |
| 4 | Xylene | 130 | 24 | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmovzh, T.N.; Rakitin, O.A. N,N-Bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)thiophen-2-amine. Molbank 2021, 2021, M1290. https://doi.org/10.3390/M1290
Chmovzh TN, Rakitin OA. N,N-Bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)thiophen-2-amine. Molbank. 2021; 2021(4):M1290. https://doi.org/10.3390/M1290
Chicago/Turabian StyleChmovzh, Timofey N., and Oleg A. Rakitin. 2021. "N,N-Bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)thiophen-2-amine" Molbank 2021, no. 4: M1290. https://doi.org/10.3390/M1290
APA StyleChmovzh, T. N., & Rakitin, O. A. (2021). N,N-Bis(4′-(hexyloxy)-[1,1′-biphenyl]-4-yl)thiophen-2-amine. Molbank, 2021(4), M1290. https://doi.org/10.3390/M1290