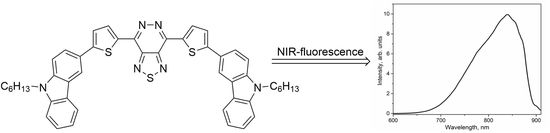
4,7-Bis(5-(9-hexyl-9H-carbazol-3-yl)thiophen-2-yl)-[1,2,5]thiadiazolo[3,4-d]pyridazine
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cheng, Y.J.; Yang, S.H.; Hsu, C.S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Molecular design of photovoltaic materials for polymer solar cells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Zeng, S.; Yin, L.; Ji, C.; Jiang, X.; Li, K.; Li, Y.; Wang, Y. D–π–A–π–D type benzothiadiazole–triphenylamine based small molecules containing cyano on the π-bridge for solution-processed organic solar cells with high open-circuit voltage. Chem. Commun. 2012, 48, 10627–10629. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.R.; Ixta, L.A.; Rodríguez, M.; Ortíz, G.R.; Maldonado, J.L.; Sánchez, A.J.; Farfán, N.; Santillan, R. Synthesis, chemical–optical characterization and solvent interaction effect of novel fluorene-chromophores with D–A–D structure. Dyes Pigm. 2013, 98, 31–41. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.M.; Chen, C.; Chen, Q.; Dou, L.; Hong, Z.; Li, G.; Yang, Y. Solution-processed small molecules using different electron linkers for high-performance solar cells. Adv. Mater. 2013, 25, 4657–4662. [Google Scholar] [CrossRef] [PubMed]
- Rakitin, O.A. Recent developments in the synthesis of 1,2,5-thiadiazoles and 2,1,3-benzothiadiazoles. Synthesis 2019, 51, 4338–4347. [Google Scholar] [CrossRef]
- Rakitin, O.A. Fused 1,2,5-thia- and 1,2,5-selenadiazoles: Synthesis and application in materials chemistry. Tetrahedron Lett. 2020, 61, 152230. [Google Scholar] [CrossRef]
- Rakitin, O.A.; Zibarev, A.V. Recent progress in synthesis and applications of 5-membered chalcogen-nitrogen π-heterocycles with three heteroatoms. Asian J. Org. Chem. 2018, 7, 2397–2416. [Google Scholar] [CrossRef]
- Paramasivam, M.; Gupta, A.; Raynor, A.M.; Bhosale, S.V.; Bhanuprakash, K.; Rao, V.J. Small band gap D-p-A-p-D benzothiadiazole derivatives with low-lying HOMO levels as potential donors for applications in organic photovoltaics: A combined experimental and theoretical investigation. RSC Adv. 2014, 4, 35318. [Google Scholar] [CrossRef]
- Castro, E.; Cabrera-Espinoza, A.; Deemer, E.; Echegoyen, L. Low-energy-gap organic based acceptor–donor–acceptor π-conjugated small molecules for bulk-heterojunction organic solar cells. Eur. J. Org. Chem. 2015, 4629–4634. [Google Scholar] [CrossRef]
- Eroglu, D.; Ergun, E.G.C.; Önal, A.M. Cross-exchange of donor units in donor-acceptor-donor type conjugated molecules: Effect of symmetrical and unsymmetrical linkage on the electrochemical and optical properties. Tetrahedron 2020, 76, 131164. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Lyssenko, K.A.; Popov, V.V.; Rakitin, O.A. Safe synthesis of 4,7-dibromo[1,2,5]thiadiazolo[3,4-d]pyridazine and its SNAr reactions. Molecules 2018, 23, 2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmovzh, T.N.; Knyazeva, E.A.; Mikhalchenko, L.V.; Golovanov, I.S.; Amelichev, S.A.; Rakitin, O.A. Synthesis of 4,7-dibromo derivative of ultrahigh electron-deficient [1,2,5]thiadiazolo[3,4-d]pyridazine heterocycle and its cross-coupling reactions. Eur. J. Org. Chem. 2018, 41, 5668–5677. [Google Scholar] [CrossRef]
- Zampetti, A.; Minotto, A.; Cacialli, F. Near-infrared (NIR) organic light-emitting diodes (OLEDs): Challenges and opportunities. Adv. Funct. Mater. 2019, 29, 1807623. [Google Scholar] [CrossRef]
- Feng, G.L.; Ji, S.J.; Lai, W.Y.; Huang, W. Synthesis and optical properties of starburst carbazoles based on 9-phenylcarbazole core. Synlett 2006, 17, 2841–2845. [Google Scholar] [CrossRef]

| Entry | Brominating Agent | Solvent | Temperature, °C | Time, h | Yield of 3, % |
|---|---|---|---|---|---|
| 1 | NBS | CHCl3 | 25 | 15 | 10 |
| 2 | NBS | CHCl3 | 60 | 6 | 8 |
| 3 | NBS | DMF | 25 | 48 | 25 |
| 4 | Br2∙dioxane | CHCl3 | 25 | 48 | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmovzh, T.N.; Korshunov, V.M.; Taydakov, I.V.; Rakitin, O.A. 4,7-Bis(5-(9-hexyl-9H-carbazol-3-yl)thiophen-2-yl)-[1,2,5]thiadiazolo[3,4-d]pyridazine. Molbank 2022, 2022, M1332. https://doi.org/10.3390/M1332
Chmovzh TN, Korshunov VM, Taydakov IV, Rakitin OA. 4,7-Bis(5-(9-hexyl-9H-carbazol-3-yl)thiophen-2-yl)-[1,2,5]thiadiazolo[3,4-d]pyridazine. Molbank. 2022; 2022(1):M1332. https://doi.org/10.3390/M1332
Chicago/Turabian StyleChmovzh, Timofey N., Vladislav M. Korshunov, Ilya V. Taydakov, and Oleg A. Rakitin. 2022. "4,7-Bis(5-(9-hexyl-9H-carbazol-3-yl)thiophen-2-yl)-[1,2,5]thiadiazolo[3,4-d]pyridazine" Molbank 2022, no. 1: M1332. https://doi.org/10.3390/M1332
APA StyleChmovzh, T. N., Korshunov, V. M., Taydakov, I. V., & Rakitin, O. A. (2022). 4,7-Bis(5-(9-hexyl-9H-carbazol-3-yl)thiophen-2-yl)-[1,2,5]thiadiazolo[3,4-d]pyridazine. Molbank, 2022(1), M1332. https://doi.org/10.3390/M1332