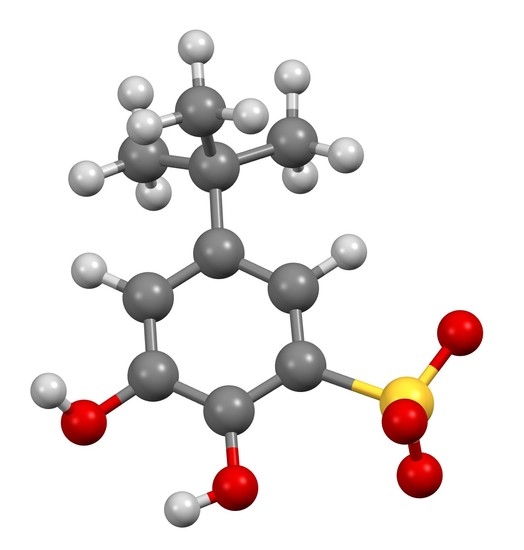
Barium 5-(tert-butyl)-2,3-dihydroxybenzenesulfonate
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Consideration
3.2. Synthesis of Barium 5-(tert-butyl)-2,3-dihydroxybenzenesulfonate
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pierpont, C.G.; Buchanan, R.M. Transition metal complexes of o-benzoquinone, o-semiquinone, and catecholate ligands. Coord. Chem. Rev. 1981, 38, 45–87. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Lhenry, S.; Leroux, Y.R.; Hapiot, P. Use of catechol as selective redox mediator in scanning electrochemical microscopy investigations. Anal. Chem. 2012, 84, 7518–7524. [Google Scholar] [CrossRef] [PubMed]
- Kemmere, M.F.; Mayer, M.J.J.; Meuldijk, J.; Drinkenburg, A.A.H. The influence of 4-tert-butylcatechol on the emulsion polymerization of styrene. J. Appl. Polym. Sci. 1999, 71, 2419–2422. [Google Scholar] [CrossRef]
- North, M.A.; Del Grosso, C.A.; Wilker, J.J. High Strength Underwater Bonding with Polymer Mimics of Mussel Adhesive Proteins. ACS Appl. Mater. Interfaces 2017, 9, 7866–7872. [Google Scholar] [CrossRef] [PubMed]
- Holten-Andersen, N.; Mates, T.E.; Toprak, M.S.; Stucky, G.D.; Zok, F.W.; Waite, J.H. Metals and the integrity of a biological coating: The cuticle of mussel byssus. Langmuir 2009, 25, 3323–3326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukyanov, D.A.; Apraksin, R.V.; Yankin, A.N.; Vlasov, P.S.; Levin, O.V.; Tolstopjatova, E.G.; Kondratiev, V.V. Synthesis and electrochemical properties of poly(3,4-dihydroxystyrene) and its composites with conducting polymers. Synth. Met. 2019, 256, 116151. [Google Scholar] [CrossRef]
- Patil, N.; Mavrandonakis, A.; Jérôme, C.; Detrembleur, C.; Palma, J.; Marcilla, R. Polymers Bearing Catechol Pendants as Universal Hosts for Aqueous Rechargeable H+, Li-Ion, and Post-Li-ion (Mono-, Di-, and Trivalent) Batteries. ACS Appl. Energy Mater. 2019, 2, 3035–3041. [Google Scholar] [CrossRef]
- Pognon, G.; Cougnon, C.; Mayilukila, D.; Belanger, D. Catechol-modified activated carbon prepared by the diazonium chemistry for application as active electrode material in electrochemical capacitor. ACS Appl. Mater. Interfaces 2012, 4, 3788–3796. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.; Jérôme, C.; Detrembleur, C. Recent advances in the synthesis of catechol-derived (bio)polymers for applications in energy storage and environment. Prog. Polym. Sci. 2018, 82, 34–91. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, Y.-H.; Cheng, J.; Cao, G.-P.; Yang, Y.-S. A study of tiron in aqueous solutions for redox flow battery application. Electrochim. Acta 2010, 55, 715–720. [Google Scholar] [CrossRef]
- Apraksin, R.V.; Volosatova, Y.A.; Volkov, A.I.; Vlasov, P.S.; Lukyanov, D.A.; Kulikov, I.R.; Eliseeva, S.N.; Levin, O.V. Electrochemical synthesis and characterization of poly [Ni(CH3Osalen)] with immobilized poly(styrenesulfonate) anion dopants. Electrochim. Acta 2021, 368, 137637. [Google Scholar] [CrossRef]
- Cerfontain, H.; Coenjaarts, N.J.; Koeberg-Telder, A. Sulfonation and sulfation on reaction of 1,2-dihydroxybenzene and its methyl ethers in concentrated aqueous sulfuric acid. Recl. Trav. Chim. Pays-Bas 2010, 107, 325–330. [Google Scholar] [CrossRef]
- Lukyanov, D.A.; Vereshchagin, A.A.; Soloviova, A.V.; Grigorova, O.V.; Vlasov, P.S.; Levin, O.V. Sulfonated Polycatechol Immobilized in a Conductive Polymer for Enhanced Energy Storage. ACS Appl. Energy Mater. 2021, 4, 5070–5078. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubicheva, L.G.; Lukyanov, D.A. Barium 5-(tert-butyl)-2,3-dihydroxybenzenesulfonate. Molbank 2022, 2022, M1336. https://doi.org/10.3390/M1336
Rubicheva LG, Lukyanov DA. Barium 5-(tert-butyl)-2,3-dihydroxybenzenesulfonate. Molbank. 2022; 2022(1):M1336. https://doi.org/10.3390/M1336
Chicago/Turabian StyleRubicheva, Lyubov G., and Daniil A. Lukyanov. 2022. "Barium 5-(tert-butyl)-2,3-dihydroxybenzenesulfonate" Molbank 2022, no. 1: M1336. https://doi.org/10.3390/M1336
APA StyleRubicheva, L. G., & Lukyanov, D. A. (2022). Barium 5-(tert-butyl)-2,3-dihydroxybenzenesulfonate. Molbank, 2022(1), M1336. https://doi.org/10.3390/M1336