Synthesis of 5-Aroyl-2-aryl-3-hydroxypyridin-4(1H)-ones
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
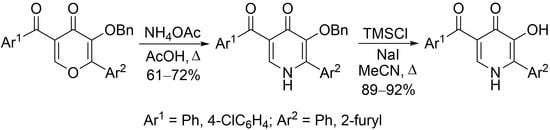
3.1. General Procedure for the Synthesis of 3-Benzyloxypyridin-4(1H)-ones 2
- 5-Benzoyl-3-(benzyloxy)-2-(furan-2-yl)pyridin-4(1H)-one (2a). Brown solid (68 mg, 68%), mp 190–191 °C. IR (ATR) ν 2879, 2854, 1655, 1614, 1540, 1314, 1255, 1188, 999, and 743 cm−1. 1H NMR (500 MHz, DMSO-d6) δ 5.30 (s, 2H, CH2), 6.74 (dd, J = 3.4, J = 1.7, 1H, H-4 furan), 7.17 (d, J = 3.4, 1H, H-3 furan), 7.31–7.39 (m, 3H, Ph), 7.41 (d, J = 7.0, 2H, H-2, H-6 Ph), 7.50 (t, J = 7.7, 2H, H-3, H-5 Ph), 7.61 (t, J = 7.4, 1H, H-4 Ph), 7.72 (d, J = 7.2, 2H, H-2, H-6 Ph), 7.79 (s, 1H, H-6 pyridone), 8.01 (d, J = 1.0, 1H, H-5 furan), and 12.14 (s, 1H, NH). 13C NMR (126 MHz, DMSO-d6) δ 71.5 (CH2), 112.9, 114.1, 125.3, 126.4, 128.1, 128.16, 128.2, 128.6, 128.9, 129.1, 129.6, 132.7, 137.0, 137.6, 143.6, 144.6, 171.2 (C=O), and 194.1 (C=O). HRMS (ESI): calculated for C23H18NO4 [M + H]+ 372.1236, found 372.1249.
- 3-(Benzyloxy)-5-(4-chlorobenzoyl)-2-(furan-2-yl)pyridin-4(1H)-one (2b). Brown solid (79 mg, 72%), mp 194–195 °C. IR (ATR) ν 2779, 2682, 1647, 1616, 1536, 1191, 1008, and 794 cm−1. 1H NMR (500 MHz, DMSO-d6) δ 5.28 (s, 2H, CH2), 6.75 (dd, J = 3.4, J = 1.8, 1H, H-4 furan), 7.16 (d, J = 3.4, 1H, H-3 furan), 7.33 (tt, J = 7.2, J = 1.4, 1H, H-4 Ph), 7.37 (t, J = 7.2, 2H, H-3, H-5 Ph), 7.41 (d, J = 7.0, 2H, H-2, H-6 Ph), 7.56 (d, J = 8.5, 2H, H-3, H-5 Ar), 7.72 (d, J = 8.5, 2H, H-2, H-6 Ar), 7.83 (s, 1H, H-6 pyridone), 8.01 (d, J = 1.0, 1H, H-5 furan), and 12.21 (s, 1H, NH). 13C NMR (126 MHz, DMSO-d6) δ 71.6 (CH2), 112.9, 114.1, 125.7, 128.1, 128.2, 128.2, 128.5, 130.9, 136.4, 137.0, 137.4, 138.2, 143.5, 143.7, 144.6, 171.2 (C=O), and 193.0 (C=O). HRMS (ESI): calculated for C23H17ClNO4 [M + H]+ 406.0846, found 406.0853.
- 3-(Benzyloxy)-5-(4-chlorobenzoyl)-2-phenylpyridin-4(1H)-one (2c). Brown solid (68 mg, 61%), mp 160–161 °C. IR (ATR) ν 3019, 2866, 1657, 1613, 1535, 1190, and 752 cm−1. 1H NMR (500 MHz, DMSO-d6) δ 5.03 (s, 2H, CH2), 7.10–7.19 (m, 2H, Ph), 7.21–7.26 (m, 3H, Ph), 7.49–7.53 (m, 3H, Ph), 7.54–7.57 (m, 2H, Ph), 7.58 (d, J = 8.5, 2H, H-3, H-5 Ar), 7.77 (d, J = 8.5, 2H, H-2, H-6 Ar), 7.90 (d, J = 6.2, 1H, H-6 pyridone), and 12.18 (d, J = 6.2, 1H, NH). 13C NMR (126 MHz, DMSO-d6) δ 72.1 (CH2), 126.5, 127.7, 128.0, 128.1, 128.25, 128.33, 129.0, 129.6, 131.0, 136.4, 137.1, 138.0, 140.3, 145.5, 171.6 (C=O), and 193.3 (C=O). HRMS (ESI): calculated for C25H18ClNO3 [M + H]+ 416.1053, found 416.1042.
3.2. General Procedure for the Synthesis of 3-Hydroxypyridin-4(1H)-ones 3
- 5-Benzoyl-2-(furan-2-yl)-3-hydroxypyridin-4(1H)-one (3a). Brown solid (63 mg, 89%), mp 234–235 °C. IR (ATR) ν 3334, 3054, 1600, 1482, 1233, and 746 cm−1. 1H NMR (500 MHz, DMSO-d6) δ 6.77 (dd, J = 3.3, J = 1.3, 1H, H-4 furan), 7.13 (d, J = 3.3, 1H, H-3 furan), 7.46 (t, J = 7.6, 2H, H-3, H-5 Ph), 7.59 (t, J = 7.3, 1H, H-4 Ph), 7.75 (d, J = 7.5, 2H, H-2, H-6 Ph), 7.78 (d, J = 3.6, 1H, H-6 pyridone), 7.99 (s, 1H, H-5 furan), and 12.24 (s, 1H, NH); the OH proton was not observed. 13C NMR (126 MHz, DMSO-d6) δ 112.5, 112.8, 119.2, 121.2, 128.0, 129.2, 132.4, 136.6, 137.9, 143.8, 144.1, 144.5, 168.6 (C=O), and 193.7 (C=O). HRMS (ESI): calculated for C16H12NO4 [M + H]+ 282.0766, found 282.0764.
- 5-(4-Chlorobenzoyl)-2-(furan-2-yl)-3-hydroxypyridin-4(1H)-one (3b). Brown solid (72 mg, 92%), mp 298–299 °C. IR (ATR) ν 3311, 3060, 2958, 1648, 1523, 1405, 1273, and 736 cm−1. 1H NMR (500 MHz, DMSO-d6) δ 6.77 (dd, J = 3.1, J = 1.6, 1H, H-4 furan), 7.13 (d, J = 3.3, 1H, H-3 furan), 7.53 (d, J = 8.4, 2H, H-3, H-5 Ar), 7.75 (d, J = 8.4, 2H, H-2, H-6 Ar), 7.81 (d, J = 6.6, 1H, H-6 pyridone), 7.99 (s, 1H, H-5 furan), and 12.30 (d, J = 6.6, 1H, NH); the OH proton was not observed. 13C NMR (126 MHz, DMSO-d6) δ 112.6, 112.9, 119.3, 120.8, 128.1, 131.1, 136.7, 137.0, 137.2, 143.8, 144.1, 144.7, 168.7 (C=O), and 192.6 (C=O). HRMS (ESI): calculated for C16H10ClNO4 [M + H]+ 316.0377, found 316.0376.
- 5-(4-Chlorobenzoyl)-3-hydroxy-2-phenylpyridin-4(1H)-one (3c). Brown solid (75 mg, 92%), mp 292–293 °C. IR (ATR) ν 3234, 3070, 1641, 1594, 1379, 1263, 1087, and 752 cm−1. 1H NMR (500 MHz, DMSO-d6) δ 7.48 (tt, J = 7.3, J = 1.2, 1H, H-4 Ph), 7.52–7.57 (m, 4H, Ph, Ar), 7.75–7.79 (m, 4H, Ar, Ph), 7.88 (d, J = 6.1, 1H, H-6 pyridone), 8.40–9.40 (br s, 1H, OH), and 12.22 (d, J = 6.1, 1H, NH). 13C NMR (126 MHz, DMSO-d6) δ 120.8, 127.4, 128.1, 128.40, 128.43, 129.1, 131.16, 131.19, 136.6, 136.9, 137.3, 146.1, 169.0 (C=O), and 192.7 (C=O). HRMS (ESI): calculated for C18H12ClNO3 [M + H]+ 326.0584, found 326.0599.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, M.A.; Marques, S.M.; Chaves, S. Hydroxypyridinones as “Privileged” Chelating Structures for the Design of Medicinal Drugs. Coord. Chem. Rev. 2012, 256, 240–259. [Google Scholar] [CrossRef]
- Santos, M.A.; Irto, A.; Buglyó, P.; Chaves, S. Hydroxypyridinone-Based Metal Chelators towards Ecotoxicity: Remediation and Biological Mechanisms. Molecules 2022, 27, 1966. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Tian, Y.; Ma, Y. Preparation of 5-Functionalised Pyridine Derivatives Using a Br/Mg Exchange Reaction: Application to the Synthesis of an Iron-Chelator Prodrug. J. Chem. Res. 2017, 41, 627–630. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, L.; Shen, L. Hydroxypyridinones as a very Promising Platform for Targeted Diagnostic and Therapeutic Radiopharmaceuticals. Molecules 2021, 26, 6997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yuan, M.; Zhang, M.; Wang, M.; Chen, J.; Li, R.; Qiu, L.; Feng, X.; Hu, J.; Wu, G. Efficient Removal of Uranium from Diluted Aqueous Solution with Hydroxypyridone Functionalized Polyethylene Nonwoven Fabrics. Radiat. Phys. Chem. 2020, 171, 108742. [Google Scholar] [CrossRef]
- Feistner, G.; Budzikiewicz, H. On the Structure of Rubrifacine. Can. J. Chem. 1985, 63, 495–499. [Google Scholar] [CrossRef]
- Adams, R.; Cristol, S.J.; Anderson, A.A.; Albert, A.A. The Structure of Leucenol. I. J. Am. Chem. Soc. 1945, 67, 89–92. [Google Scholar] [CrossRef]
- Chen, A.Y.; Adamek, R.N.; Dick, B.L.; Credille, C.V.; Morrison, C.N.; Cohen, S.M. Targeting Metalloenzymes for Therapeutic Intervention. Chem. Rev. 2019, 119, 1323–1455. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Lazerwith, S.E.; Pyun, H.-J. Polycyclic-Carbamoylpyridone Compounds and Their Pharmaceutical Use. U.S. Patent US9700554, 11 July 2017. [Google Scholar]
- Miyazaki, S.; Isoshima, H.; Oshita, K.; Kawashita, S.; Nagahashi, N.; Terashita, M. Substituted Spiropyrido[1,2-a]Pyrazine Derivative and Medicinal Use Thereof as HIV Integrase Inhibitor. U.S. Patent US10087178, 2 October 2018. [Google Scholar]
- Kankanala, J.; Kirby, K.A.; Liu, F.; Miller, L.; Nagy, E.; Wilson, D.J.; Parniak, M.A.; Sarafianos, S.G.; Wang, Z. Design, Synthesis, and Biological Evaluations of Hydroxypyridonecarboxylic Acids as Inhibitors of HIV Reverse Transcriptase Associated RNase H. J. Med. Chem. 2016, 59, 5051–5062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasuji, T.; Johns, B.A.; Yoshida, H.; Taishi, T.; Taoda, Y.; Murai, H.; Kiyama, R.; Fuji, M.; Yoshinaga, T.; Seki, T.; et al. Carbamoyl Pyridone HIV-1 Integrase Inhibitors. 1. Molecular Design and Establishment of an Advanced Two-Metal Binding Pharmacophore. J. Med. Chem. 2012, 55, 8735–8744. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Sirous, H.; Zabihollahi, R.; Aghasadeghi, M.R.; Sadat, S.M.; Namazi, R.; Saghaie, L.; Memarian, H.R.; Fassihi, A. Design, Synthesis and Anti-HIV-1 Evaluation of a Series of 5-Hydroxypyridine-4-One Derivatives as Possible Integrase Inhibitors. Med. Chem. Res. 2015, 24, 4113–4127. [Google Scholar] [CrossRef]
- Sayyed, S.K.; Quraishi, M.; Jobby, R.; Rameshkumar, N.; Kayalvizhi, N.; Krishnan, M.; Sonawane, T. A Computational Overview of Integrase Strand Transfer Inhibitors (INSTIs) against Emerging and Evolving Drug-Resistant HIV-1 Integrase Mutants. Arch. Microbiol. 2023, 205, 142. [Google Scholar] [CrossRef] [PubMed]
- Sirous, H.; Fassihi, A.; Brogi, S.; Campiani, G.; Christ, F.; Debyser, Z.; Gemma, S.; Butini, S.; Chemi, G.; Grillo, A.; et al. Synthesis, Molecular Modelling and Biological Studies of 3-Hydroxypyrane-4-One and 3-Hydroxy-Pyridine-4-One Derivatives as HIV-1 Integrase Inhibitors. Med. Chem. 2019, 15, 755–770. [Google Scholar] [CrossRef] [PubMed]
- De Beer, J.; Petzer, J.P.; Lourens, A.C.U.; Petzer, A. Design, Synthesis and Evaluation of 3-Hydroxypyridin-4-Ones as Inhibitors of Catechol-O-Methyltransferase. Mol. Divers. 2021, 25, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Senaweera, S.; Edwards, T.C.; Kankanala, J.; Wang, Y.; Sahani, R.L.; Xie, J.; Geraghty, R.J.; Wang, Z. Discovery of N-Benzyl Hydroxypyridone Carboxamides as a Novel and Potent Antiviral Chemotype against Human Cytomegalovirus (HCMV). Acta Pharm. Sin. B 2022, 12, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Fujishita, T.; Mikamiyama, M.; Kawai, M.; Akiyama, T. Substituted 3-Hydroxy-4-Pyridone Derivative. U.S. Patent US8835461, 16 September 2014. [Google Scholar]
- Ma, Y.; Hider, R.C. Novel Synthetic Approach to Fluoro- and Amido-Disubstituted 3-Hydroxypyridin-4-Ones. J. Fluor. Chem. 2015, 173, 29–34. [Google Scholar] [CrossRef]
- Obydennov, D.L.; Viktorova, V.V.; Chernyshova, E.V.; Shirinkin, A.S.; Usachev, S.A.; Sosnovskikh, V.Y. Direct Synthesis of 5-Acyl-3-Oxy-4-Pyrones Based On Acid-Catalyzed Acylation of Enaminodiones with Acylbenzotriazoles via Soft Enolization. Synthesis 2020, 52, 2267–2276. [Google Scholar] [CrossRef]
- Obydennov, D.L.; Khammatova, L.R.; Steben’kov, V.D.; Sosnovskikh, V.Y. Synthesis of novel polycarbonyl Schiff bases by ring-opening reaction of ethyl 5-acyl-4-pyrone-2-carboxylates with primary mono- and diamines. RSC Advances 2019, 9, 40072–40083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obydennov, D.L.; Simbirtseva, A.E.; Piksin, S.E.; Sosnovskikh, V.Y. 2,6-Dicyano-4-pyrone as a Novel and Multifarious Building Block for the Synthesis of 2,6-Bis(hetaryl)-4-pyrones and 2,6-Bis(hetaryl)-4-pyridinols. ACS Omega 2020, 5, 33406–33420. [Google Scholar] [CrossRef] [PubMed]
- Usachev, S.A.; Nigamatova, D.I.; Mysik, D.K.; Naumov, N.A.; Obydennov, D.L.; Sosnovskikh, V.Y. 2-Aryl-6-Polyfluoroalkyl-4-Pyrones as Promising RF-Building-Blocks: Synthesis and Application for Construction of Fluorinated Azaheterocycles. Molecules 2021, 26, 4415. [Google Scholar] [CrossRef] [PubMed]


| Entry | Compounds 1–3 | R1 | R2 | Yield of 2 | Yield of 3 |
|---|---|---|---|---|---|
| 1 | a | Ph | furan-2-yl | 68 | 89 |
| 2 | b | 4-ClC6H4 | furan-2-yl | 72 | 92 |
| 3 | c | 4-ClC6H4 | Ph | 61 | 92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steparuk, E.V.; Obydennov, D.L.; Sosnovskikh, V.Y. Synthesis of 5-Aroyl-2-aryl-3-hydroxypyridin-4(1H)-ones. Molbank 2023, 2023, M1668. https://doi.org/10.3390/M1668
Steparuk EV, Obydennov DL, Sosnovskikh VY. Synthesis of 5-Aroyl-2-aryl-3-hydroxypyridin-4(1H)-ones. Molbank. 2023; 2023(2):M1668. https://doi.org/10.3390/M1668
Chicago/Turabian StyleSteparuk, Elena V., Dmitrii L. Obydennov, and Vyacheslav Y. Sosnovskikh. 2023. "Synthesis of 5-Aroyl-2-aryl-3-hydroxypyridin-4(1H)-ones" Molbank 2023, no. 2: M1668. https://doi.org/10.3390/M1668
APA StyleSteparuk, E. V., Obydennov, D. L., & Sosnovskikh, V. Y. (2023). Synthesis of 5-Aroyl-2-aryl-3-hydroxypyridin-4(1H)-ones. Molbank, 2023(2), M1668. https://doi.org/10.3390/M1668