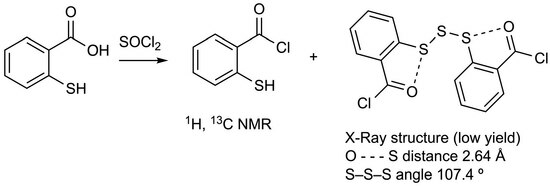
2,2′-Trisulfanediyldibenzoyl Chloride
Abstract
:1. Introduction
2. Results
| O…S Distance | O…S–X Angles | CSD Refcode | Ref | |
|---|---|---|---|---|
| 5 | 2.641(3) | 173.46 | — | This work |
| 3 * | 2.653(3), 2.664(3) | 171.78(8), 173.99(8) | VEPDAV | [16] |
| 3 * | 2.654(3) | 177.19(8) | VEPDAV | [16] |
| 13 | 2.941(4) 2.727(3) | 77.1(1), 173.7(2) | VEPCUO | [16] |
| 14 | 2.700(1) | 169.65(6) | TAJFIV | [18] |
| 15 | 2.670(4) | 160.0(2) | SIPTEQ | [19] |
3. Experimental
3.1. Preparation of 2-Mercaptobenzoyl Chloride to Obtain NMR Data
3.2. Formation and X-ray Structure Determination of 2,2′-Trisulfanediyldibenzoyl Chloride 5
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McElvain, S.M.; Carney, T.P. Piperidine derivatives XVII. Local anesthetics derived from substituted piperidinoalcohols. J. Am. Chem. Soc. 1946, 68, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, E.; Hanus, H.D. Synthesen von Heterocyclen 65. Mitt.: Über Reaktionen mit Salicylsäurechlorid. Monatsh. Chem. 1965, 96, 411–417. [Google Scholar] [CrossRef]
- Hirokazu, T.; Takayuki, K.; Naoki, I. Reactions of thiobenzophenones with benzenediazonium-o-carboxylate, and salicyloyl and o-mercaptobenzoyl chlorides as 1,4-dipolar reagents. Bull. Chem. Soc. Jpn. 1972, 45, 2220–2221. [Google Scholar] [CrossRef]
- Wang, S.; Fang, K.; Dong, G.; Chen, S.; Liu, N.; Miao, Z.; Yao, J.; Li, J.; Zhang, W.; Sheng, C. Scaffold diversity inspired by the natural product evodiamine: Discovery of highly potent and multitargeting antitumor agents. J. Med. Chem. 2015, 58, 6678–6696. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Rani, S.; Arora, A.; Aulakh, D.; Wriedt, M. Thioester-appended organosilatranes: Synthetic investigations and application in the modification of magnetic silica surfaces. New J. Chem. 2016, 40, 6200–6213. [Google Scholar] [CrossRef]
- Neamati, N.; Lin, Z.; Karki, R.G.; Orr, A.; Cowansage, K.; Strumberg, D.; Pais, G.C.G.; Voigt, J.H.; Nicklaus, M.C.; Winslow, H.E.; et al. Metal-dependent inhibition of HIV-1 integrase. J. Med. Chem. 2002, 45, 5661–5670. [Google Scholar] [CrossRef]
- Sengar, R.S.; Miller, J.J.; Basu, P. Design, synthesis, and characterisation of dioxo-molybdenum(VI) complexes with thiolate ligands: Effects of intraligand NH…S hydrogen bonding. Dalton Trans. 2008, 2569–2577. [Google Scholar] [CrossRef]
- Shukla, R.; Dhaka, A.; Aubert, E.; Vijayakumar-Syamala, V.; Jeannin, O.; Fourmigué, M.; Espinosa, E. Understanding reactivity and assembly of dichalcogenides: Structural, electrostatic potential, and topological analyses of 3H-1,2-benzodithiol-3-one and selenium analogs. Cryst. Growth Des. 2020, 20, 7704–7725. [Google Scholar] [CrossRef]
- Elschenbroich, C.; Lu, F.; Harms, K.; Burghaus, O.; Pietzonka, C.; Pebler, J. α,ω-Di([5]trovacenyl) sulfides TVC–Sn–TVC (n = 1–4) and TVC–SCH2S–TVC: A study in intramolecular communication. Eur. J. Inorg. Chem. 2012, 2012, 3929–3936. [Google Scholar] [CrossRef]
- Bhattacherjee, D.; Sufian, A.; Mahato, S.K.; Begum, S.; Banerjee, K.; De, S.; Srivastava, H.K.; Bhabak, K.P. Trisulfides over disulfides: Highly selective synthetic strategies, anti-proliferative activities and sustained H2S release profiles. Chem. Commun. 2019, 55, 13534–13537. [Google Scholar] [CrossRef]
- Ostrowski, M.; Jeske, J.; Jones, P.G.; du Mont, W.-W. Eigenschaften von Chalcogen–Chalcogen -Bindungen, XVII. Di- und Trisulfane mit sterisch anspruchvollen Alkyl-Substituenten: Das erste trans 1-Dialkyldisulfan. Chem. Ber. 1993, 126, 1355–1359. [Google Scholar] [CrossRef]
- Linden, A.; Majchrzak, A.; Cavegn, J.; Mloston, G.; Heimgartner, H. Four bis(1-chloro-2,2,4,4-tetramethyl-3-ococyclobutan-1-yl)oligosulfanes. Acta Crystallogr. Sect. C 2002, 58, o480–o484. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Mootz, D.; Bäck, B.; Minkwitz, R. Bis(trifluoromethyl)disulfan und -trisulfan: Molekülgeometrie im festen Zustand. Z. Naturforch. B 1997, 52, 69–71. [Google Scholar] [CrossRef]
- Ali, B.; Dance, I.; Scudder, M.; Craig, D. Principles of crystal packing and intermolecular motifs for Ph3XSnXPh3, X = C, Si, Ge, Sn. Cryst. Eng. Comm. 2001, 28, 120–127. [Google Scholar] [CrossRef]
- Brisse, F.; Vanier, M.; Olivier, M.J.; Gareau, Y.; Steliou, K. Crystal structures of (μ-trithio)bis[tricyclohexylgermanium(IV)] and (μ-trithio)bis[triphenylgermanium(IV)]. Organometallics 1983, 2, 878–882. [Google Scholar] [CrossRef]
- Párkányi, L.; Kálmán, A.; Kucsman, Á.; Kapovits, I. Intramolecular sulphur(II)–oxygen interaction in acyl chlorides: An X-ray study of 2,2’-thiodibenzoyl chloride and 2,2’-dithiodibenzoyl chloride. J. Mol. Struct. 1979, 198, 355–364. [Google Scholar] [CrossRef]
- Gleiter, R.; Haberahuer, G.; Werz, D.B.; Rominger, F.; Bleiholder, C. From noncovalent chalcogen-chalcogen interactions to supramolecular aggregates: Experiments and calculations. Chem. Rev. 2018, 118, 2010–2041. [Google Scholar] [CrossRef]
- Shishkin, O.V.; Omelchenko, I.V.; Kalyuzhny, A.L.; Paponov, B.V. Intramolecular S–O chalcogen bond in thioindirubin. Struct. Chem. 2010, 21, 1005–1011. [Google Scholar] [CrossRef]
- Nagao, Y.; Hirata, T.; Goto, S.; Sano, S.; Kakehi, A.; Iizuka, K.; Motoo, S. Intramolecular nonbonded S…O interaction recognized in (acylimino)thiadiazoline derivatives as angiotensin II receptor antagonists and related compounds. J. Am. Chem. Soc. 1998, 120, 3104–3110. [Google Scholar] [CrossRef]
- Monge, A.; Martinez-Merino, V.; Fernández-Alvarez, E. Synthesis of 2,2’-dithiobis- and 2,2’-trithiobis-3-pyridinecarboxylic acid derivatives as new potential radiosensitizers/radioprotectors. J. Heterocycl. Chem. 1988, 25, 23–28. [Google Scholar] [CrossRef]
- Ogata, M.; Matsumoto, H.; Shimizu, S. Reaction of N,N’-thionyldiimidazole with thiols: A sulfur transfer reaction. Heterocycles 1980, 14, 955–958. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]






| Compd | CSD Refcode | S–S Length (Å) | S–S–S Angle (°) | C–S–S angle (°) | Ref |
|---|---|---|---|---|---|
| 5 | — | 2.047(1) | 107.39(7) | 104.8(1) | This work |
| 6 | LEBFOP | 2.058(2) | 105.3(1) | 103.85(14) | [9] |
| 7 | NUBYIV | 2.023(4), 2.021(4) | 106.9(1) | 103.5(3), 103.3(3) | [10] |
| 8 | PEKYOT | 2.066(1), 2.057(1) | 112.54(3) | 112.05(7), 111.15(7) | [11] |
| 9 * | XOSBEM | 2.0503(5), 2.0426(5) | 107.64(2) | 103.63(5), 101.82(5) | [12] |
| 9 * | XOSBEM | 2.0513(5), 2.0418(6) | 107.99(2) | 103.61(5), 101.41(5) | [12] |
| 10 | RIKXIS | 2.0412 | 106.75 | 99.93 | [13] |
| 11 | UBIVUW | 2.047(1), 2.039(2) | 110.53(5) | 107.1(1), 105.6(1) | [14] |
| 12 | CAFFAQ | 2.0159, 2.0304 | 107.58 | 104.30, 102.07 § | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Campbell, A.H.; Fletcher, C.E.; Slawin, A.M.Z. 2,2′-Trisulfanediyldibenzoyl Chloride. Molbank 2023, 2023, M1731. https://doi.org/10.3390/M1731
Aitken RA, Campbell AH, Fletcher CE, Slawin AMZ. 2,2′-Trisulfanediyldibenzoyl Chloride. Molbank. 2023; 2023(3):M1731. https://doi.org/10.3390/M1731
Chicago/Turabian StyleAitken, R. Alan, Alexandra H. Campbell, Chloé E. Fletcher, and Alexandra M. Z. Slawin. 2023. "2,2′-Trisulfanediyldibenzoyl Chloride" Molbank 2023, no. 3: M1731. https://doi.org/10.3390/M1731
APA StyleAitken, R. A., Campbell, A. H., Fletcher, C. E., & Slawin, A. M. Z. (2023). 2,2′-Trisulfanediyldibenzoyl Chloride. Molbank, 2023(3), M1731. https://doi.org/10.3390/M1731