Real-Time Gas Identification by Analyzing the Transient Response of Capillary-Attached Conductive Gas Sensor
Abstract
:1. Introduction
2. Experimental Method
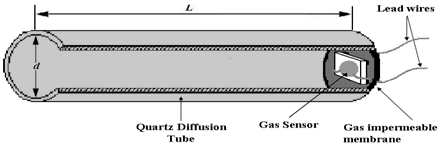
2.1. CGS Prototype Structure
2.2. Measurement System
2.3. Preprocessing
- Gs (k) is the original output of sensor.
- Gs (0) is the initial baseline output of sensor.
- ΔGs (k) is the adjusted output value of sensor.
2.4. Odor Database
2.5. Feature Extraction and Classification
- k = 11, 12, 13, … 100; indicates the number of samples in the selected portion of transient response.
- c = 1, 2, 3, 4; is the class number.
- Li is the concentration level where;
- L = [50, 100, 150, 200, 300, 400, 500, 600, 700, 800, 900, 1,000] and
- i = 1, 2, ..., 12, and
- r = 1, 2, …, 5; is the repetition number for different experiments of the same concentration in time.
- ∑B is between class covariance matrix, and
- ∑W is within class covariance matrix.
3. Result and Discussion
4. Conclusion
References
- Nanto, H.; Stetter, J.R. Introduction to chemosensors. In Handbook of Machine Olfaction: Electronic Nose Technology; Pearce, T.C., Schiffman, S.S., Nagle, H.T., Gardner, J.W., Eds.; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev 2004, 24, 181–198. [Google Scholar]
- Nakata, S.; Ojima, N. Detection of a sample gas in the presence of an interferant gas based on a nonlinear dynamic response. Sens. Actuat. B 1999, 56, 79–84. [Google Scholar]
- Nakata, S.; Akakabe, S.; Nakasuji, M.; Yoshikawa, K. Gas sensing based on a nonlinear response: Discrimination between hydrocarbons and quantification of individual components in a gas mixture. Anal. Chem 1996, 68, 2067–2072. [Google Scholar]
- Fort, A.; MacHetti, N.; Rocchi, S.; Serrano, B.; Tondi, L.; Ulivieri, N.; Vignoli, V.; Sberveglieri, G. Tin oxide gas sensing: Comparison among different measurement techniques for gas mixture classification. IEEE Trans. Instrum. Meas 2003, 52, 921–926. [Google Scholar]
- Schweizer-Berberich, M.; Zheng, J.G.; Weimar, U.; Göpel, W.; Bârsan, N.; Pentia, E.; Tomescu, A. The effect of Pt and Pd surface doping on the response of nanocrystalline tin dioxide gas sensors to CO. Sens. Actuat. B 1996, 31, 71–75. [Google Scholar]
- Williams, E.W.; Keeling, A.G. Thick film tin oxide sensors for detecting carbon monoxide at room temperature. J. Mater. Sci.: Mater. Electron 1998, 9, 51–54. [Google Scholar]
- Kim, D.H.; Lee, S.H.; Kim, K.H. Comparison of CO-gas sensing characteristics between mono- and multi-layer Pt/SnO2 thin films. Sens. Actuat. B 2001, 77, 427–431. [Google Scholar]
- Sauvan, M.; Pijolat, C. Selectivity improvement of SnO2 films by superficial metallic films. Sens. Actuat. B 1999, 58, 295–301. [Google Scholar]
- Tamaekong, N.; Liewhiran, C.; Wisitsoraat, A.; Phanichphant, S. Sensing Characteristics of Flame-Spray-Made Pt/ZnO Thick Films as H2 Gas Sensor. Sensors 2009, 9, 6652–6669. [Google Scholar]
- Safonova, O.V.; Delabouglise, G.; Chenevier, B.; Gaskov, A.M.; Labeau, M. CO and NO2 gas sensitivity of nanocrystalline tin dioxide thin films doped with Pd, Ru and Rh. Mater. Sci. Eng. C 2002, 21, 105–111. [Google Scholar]
- Safonova, O.V.; Rumyantseva, M.N.; Ryabova, L.I.; Labeau, M.; Delabouglise, G.; Gaskov, A.M. Effect of combined Pd and Cu doping on microstructure, electrical and gas sensor properties of nanocrystalline tin dioxide. Mater. Sci. Eng. B 2001, 85, 43–49. [Google Scholar]
- Zhang, G.; Liu, M. Effect of particle size and dopant on properties of SnO2-based gas sensors. Sens. Actuat. B 2000, 69, 144–152. [Google Scholar]
- Pagnier, T.; Boulova, M.; Galerie, A.; Gaskov, A.; Lucazeau, G. Reactivity of SnO2-CuO nanocrystalline materials with H2S: A coupled electrical and Raman spectroscopic study. Sens. Actuat. B 2000, 71, 134–139. [Google Scholar]
- Fukui, K.; Katsuki, A. Improvement of humidity dependence in gas sensor based on SnO2. Sens. Actuat. B 2000, 65, 316–318. [Google Scholar]
- Choi, S.D.; Lee, D.D. CH4 sensing characteristics of K-, Ca-, Mg impregnated SnO2 sensors. Sens. Actuat. B 2001, 77, 335–338. [Google Scholar]
- Ivanovskaya, M.; Bogdanov, P.; Faglia, G.; Nelli, P.; Sberveglieri, G.; Taroni, A. On the role of catalytic additives in gas-sensitivity of SnO2-Mo based thin film sensors. Sens. Actuat. B 2001, 77, 268–274. [Google Scholar]
- Han, C.-H.; Han, S.-D.; Khatkar, S. Enhancement of H2-sensing properties of F-doped SnO2 sensorby surface modification with SiO2. Sensors 2006, 6, 492–502. [Google Scholar]
- Kim, I.; Han, S.; Han, C.; Gwak, J.; Lee, H.; Wang, J. Micro semiconductor CO sensors based on indium-doped tin dioxide nanocrystalline powders. Sensors 2006, 6, 526–535. [Google Scholar]
- Kwon, C.H.; Yun, D.H.; Hong, H.K.; Kim, S.R.; Lee, K.; Lim, H.Y.; Yoon, K.H. Multi-layered thick-film gas sensor array for selective sensing by catalytic filtering technology. Sens. Actuat. B 2000, 65, 327–330. [Google Scholar]
- Hossein-Babaei, F.; Orvatinia, M. Analysis of thickness dependence of the sensitivity in thin film resistive gas sensors. Sens. Actuat. B 2003, 89, 256–261. [Google Scholar]
- Batzill, M. Surface science studies of gas sensing materials: SnO2. Sens. J 2006, 6, 1345–1366. [Google Scholar]
- Sakai, G.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N. Theory of gas-diffusion controlled sensitivity for thin film semiconductor gas sensor. Sens. Actuat. B 2001, 80, 125–131. [Google Scholar]
- Hossein-Babaei, F.; Hosseini-Golgoo, S.M.; Amini, A. Extracting discriminative information from the Padé-Z-transformed responses of a temperature-modulated chemoresistive sensor for gas recognition. Sens. Actuat. B 2009, 142, 19–27. [Google Scholar]
- Vergara, A.; Martinelli, E.; Llobet, E.; Giannini, F.; D'Amico, A.; Di Natale, C. An alternative global feature extraction of temperature modulated micro-hotplate gas sensors array using an energy vector approach. Sens. Actuat. B 2007, 124, 352–359. [Google Scholar]
- Vergara, A.; Llobet, E.; Brezmes, J.; Ivanov, P.; Cané, C.; Gràcia, I.; Vilanova, X.; Correig, X. Quantitative gas mixture analysis using temperature-modulated micro-hotplate gas sensors: Selection and validation of the optimal modulating frequencies. Sens. Actuat. B 2007, 123, 1002–1016. [Google Scholar]
- Ding, H.; Ge, H.; Liu, J. High performance of gas identification by wavelet transform-based fast feature extraction from temperature modulated semiconductor gas sensors. Sens. Actuat. B 2005, 107, 749–755. [Google Scholar]
- Sysoev, V.; Kiselev, I.; Frietsch, M.; Goschnick, J. Temperature gradient effect on gas discrimination power of a metal-oxide thin-film sensor microarray. Sensors 2004, 4, 37–46. [Google Scholar]
- Hossein-Babaei, F.; Hosseini-Golgoo, S.M. Analyzing the responses of a thermally modulated gas sensor using a linear system identification technique for gas diagnosis. IEEE Sens. J 2008, 8, 1837–1847. [Google Scholar]
- Gutierrez-Osuna, R.; Gutierrez-Galvez, A.; Powar, N. Transient response analysis for temperature-modulated chemoresistors. Sens. Actuat. B 2003, 93, 57–66. [Google Scholar]
- Gutierrez-Osuna, R.; Nagle, H.T. A method for evaluating data-preprocessing techniques for odor classification with an array of gas sensors. IEEE Trans. Syst. Man Cybern. B 1999, 29, 626–632. [Google Scholar]
- Kermani, B.G.; Schiffman, S.S.; Troy Nagle, H. Using neural networks and genetic algorithms to enhance performance in an electronic nose. IEEE Trans. Biomed. Eng 1999, 46, 429–439. [Google Scholar]
- Phaisangittisagul, E.; Nagle, H.T. Enhancing multiple classifier system performance for machine olfaction using odor-type signatures. Sens. Actuat. B 2007, 125, 246–253. [Google Scholar]
- Phaisangittisagul, E.; Nagle, H.T. Sensor selection for machine olfaction based on transient feature extraction. IEEE Trans. Insrum. Meas 2008, 57, 369–378. [Google Scholar]
- Hossein-Babaei, F.; Orvatinia, M. Gas diagnosis based on selective diffusion retardation in an air filled capillary. Sens. Actuat. B 2003, 96, 298–303. [Google Scholar]
- Hossein-Babaei, F.; Hemmati, M.; Dehmobed, M. Gas diagnosis by a quantitative assessment of the transient response of a capillary-attached gas sensor. Sens. Actuat. B 2005, 107, 461–467. [Google Scholar]
- Bahraminejad, B.; Basri, S.; Hambali, Z.; Isa, M. Single selective gas sensor for detecting flammable gases. IEICE Electron. Express 2009, 6, 876–882. [Google Scholar]
- Gutierrez-Osuna, R. Pattern analysis for machine olfaction: A review. IEEE Sens. J 2002, 2, 189–202. [Google Scholar]
- Bahraminejad, B.; Basri, S.; Hambali, Z.; Isa, M. Evaluation of dimension effects on capillary-attached gas sensor. Meas. Sci. Technol. 2010, 21, 065202:1–065202:7. [Google Scholar]
- Lee, S.W.; Tsai, P.P.; Chen, H. Comparison study of SnO2 thin- and thick-film gas sensors. Sens. Actuat. B 2000, 67, 122–127. [Google Scholar]
- El Khakani, M.A.; Dolbec, R.; Serventi, A.M.; Horrillo, M.C.; Trudeau, M.; Saint-Jacques, R.G.; Rickerby, D.G.; Sayago, I. Pulsed laser deposition of nanostructured tin oxide films for gas sensing applications. Sens. Actuat. B 2001, 77, 383–388. [Google Scholar]
- Gutierrez-Osuna, R.; Nagle, H.T.; Kermani, B.; Schiffman, S.S. Signal conditioning and pre-processing. In Handbook of Machine Olfaction: Electronic Nose Technology; Pearce, T.C., Schiffman, S.S., Nagle, H.T., Gardner, J.W., Eds.; Wiley-VCH: Weinheim, Germany, 2002; pp. 105–132. [Google Scholar]
- Hines, E.L.; Boilot, P.; Gardner, J.W.; Gongora, M.A. Pattern Analysis for Electronic Noses. In Handbook of Machine Olfaction: Electronic Nose Technology; Pearce, T.C., Schiffman, S.S., Nagle, H.T., Gardner, J.W., Eds.; Wiley-VCH: Weinheim, Germany, 2002; pp. 133–160. [Google Scholar]
- Distante, C.; Leo, M.; Siciliano, P.; Persaud, K.C. On the study of feature extraction methods for an electronic nose. Sens. Actuat. B 2002, 87, 274–288. [Google Scholar]
- Pardo, M.; Sberveglieri, G. Comparing the performance of different features in sensor arrays. Sens. Actuat. B 2007, 123, 437–443. [Google Scholar]
- Bellman, R.E. Adaptive Control Processes: A Guided Tour; Princeton University Press: Princeton, NJ, USA, 1961. [Google Scholar]
- Bishop, C.M. Neural Networks for Pattern Recognition; Oxford University: New York, NY, USA, 1995. [Google Scholar]
- Duda, R.O.; Hart, P.E.; Stork, D.G. Pattern Classification, 2nd ed; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: New York, NY, USA, 2001. [Google Scholar]
- Pardo, M.; Sberveglieri, G. Classification of electronic nose data with support vector machines. Sens. Actuat. B 2005, 107, 730–737. [Google Scholar]
- Brudzewski, K.; Osowski, S.; Markiewicz, T. Classification of milk by means of an electronic nose and SVM neural network. Sens. Actuat. B 2004, 98, 291–298. [Google Scholar]
- Distante, C.; Ancona, N.; Siciliano, P. Support vector machines for olfactory signals recognition. Sens. Actuat. B 2003, 88, 30–39. [Google Scholar]
- Demuth, H.; Beale, M. Neural Network Toolbox User’S Guide: For Use with Matlab, Version 4; MathWorks: Natick, MA, USA, 2004. [Google Scholar]
- Liang, X.; Xiaodong, W. Gas quantitative analysis with support vector machine. Proceedings of Control and Decision Conference, Guilin, China, June 17–19, 2009; pp. 5148–5151.
- Jain, A.K.; Duin, R.P.W.; Mao, J. Statistical pattern recognition: A review. IEEE Trans. Patt. Anal. Mach. Intell 2000, 22, 4–37. [Google Scholar]








| Class label | Alcohol | No. of samples in each concentration | No. of total samples | No. of samplings per sample |
|---|---|---|---|---|
| 1 | Methanol | 5 | 60 | 100 |
| 2 | Ethanol | 5 | 60 | 100 |
| 3 | 2-Propanol | 5 | 60 | 100 |
| 4 | 1-Butanol | 5 | 60 | 100 |
| Length of Sampled Feature | Feature Reduction Technique | |||||||
|---|---|---|---|---|---|---|---|---|
| PCA | LDA | |||||||
| Classifier | Classifier | |||||||
| Quadratic | KNN | ANN | SVM | Quadratic | KNN | ANN | SVM | |
| 11–15 | 57.55 | 75.00 | 65.40 | 70.26 | 68.88 | 70.50 | 75.59 | 81.20 |
| 16–20 | 55.61 | 72.00 | 63.96 | 71.80 | 71.43 | 72.30 | 76.62 | 81.83 |
| 21–25 | 56.30 | 66.49 | 66.43 | 71.30 | 77.55 | 74.90 | 82.99 | 79.10 |
| 26–30 | 58.88 | 74.20 | 68.84 | 69.64 | 82.14 | 83.88 | 82.95 | 79.93 |
| 31–35 | 57.04 | 69.43 | 69.31 | 65.92 | 92.86 | 85.31 | 91.75 | 79.76 |
| 36–40 | 57.35 | 72.24 | 71.33 | 66.53 | 95.41 | 93.67 | 94.27 | 81.27 |
| 41–45 | 58.78 | 70.53 | 70.83 | 70.03 | 99.49 | 98.27 | 98.36 | 85.80 |
| 46–50 | 61.12 | 71.88 | 71.71 | 69.13 | 99.49 | 98.27 | 98.47 | 88.87 |
| 51–55 | 62.65 | 76.04 | 71.87 | 70.35 | 100.00 | 99.49 | 98.00 | 98.50 |
| 56–60 | 63.06 | 82.90 | 72.17 | 72.06 | 100.00 | 100.00 | 100.00 | 98.83 |
| 61–65 | 62.40 | 79.96 | 70.39 | 70.45 | 100.00 | 99.59 | 99.90 | 97.30 |
| 66–70 | 62.65 | 80.50 | 72.56 | 71.31 | 100.00 | 100.00 | 99.08 | 97.06 |
| 71–75 | 62.45 | 79.59 | 71.51 | 70.75 | 100.00 | 100.00 | 99.90 | 100.00 |
| 76–80 | 62.65 | 79.47 | 73.32 | 72.70 | 100.00 | 100.00 | 100.00 | 98.60 |
| 81–85 | 63.98 | 81.67 | 73.36 | 72.08 | 100.00 | 100.00 | 99.80 | 100.00 |
| 86–90 | 64.20 | 79.84 | 73.80 | 73.16 | 100.00 | 100.00 | 99.90 | 99.09 |
| 91–95 | 63.90 | 78.73 | 73.97 | 73.56 | 100.00 | 100.00 | 100.00 | 99.50 |
| 96–100 | 64.40 | 79.96 | 74.00 | 73.70 | 100.00 | 100.00 | 100.00 | 100.00 |
| Length of Sampled Feature | Feature Reduction Technique | |||||||
|---|---|---|---|---|---|---|---|---|
| PCA | LDA | |||||||
| Classifier | Classifier | |||||||
| Quadratic | KNN | ANN | SVM | Quadratic | KNN | ANN | SVM | |
| 11–15 | 55.31 | 63.78 | 66.73 | 71.30 | 73.67 | 63.67 | 73.98 | 77.80 |
| 16–20 | 57.14 | 60.71 | 67.30 | 72.10 | 74.39 | 72.65 | 74.92 | 76.25 |
| 21–25 | 58.98 | 60.50 | 62.66 | 72.04 | 79.18 | 80.71 | 80.94 | 75.66 |
| 26–30 | 58.98 | 64.60 | 66.85 | 71.34 | 86.53 | 88.98 | 85.08 | 79.86 |
| 31–35 | 57.86 | 65.61 | 70.97 | 70.80 | 94.90 | 93.27 | 91.91 | 79.25 |
| 36–40 | 59.08 | 64.69 | 70.39 | 72.23 | 98.57 | 97.45 | 95.60 | 80.95 |
| 41–45 | 58.57 | 65.70 | 69.66 | 71.65 | 99.49 | 99.90 | 99.18 | 86.05 |
| 46–50 | 59.69 | 66.12 | 71.70 | 73.18 | 99.10 | 99.39 | 98.98 | 90.50 |
| 51–55 | 61.12 | 70.51 | 72.10 | 71.70 | 98.98 | 100.00 | 99.20 | 97.18 |
| 56–60 | 62.45 | 72.30 | 69.74 | 70.43 | 100.00 | 100.00 | 99.90 | 100.00 |
| 61–65 | 62.04 | 72.55 | 70.68 | 69.52 | 100.00 | 100.00 | 100.00 | 100.00 |
| 66–70 | 60.82 | 71.02 | 72.17 | 71.90 | 100.00 | 100.00 | 100.00 | 100.00 |
| 71–75 | 61.00 | 74.29 | 72.80 | 71.99 | 100.00 | 100.00 | 100.00 | 99.50 |
| 76–80 | 60.71 | 72.14 | 72.30 | 72.08 | 100.00 | 99.30 | 99.69 | 100.00 |
| 81–85 | 62.14 | 74.49 | 72.80 | 73.21 | 100.00 | 99.69 | 99.80 | 99.10 |
| 86–90 | 63.88 | 75.92 | 72.58 | 72.66 | 100.00 | 100.00 | 100.00 | 99.22 |
| 91–95 | 65.00 | 73.98 | 71.22 | 74.06 | 100.00 | 100.00 | 99.70 | 100.00 |
| 96–100 | 65.00 | 75.20 | 74.24 | 72.07 | 100.00 | 100.00 | 100.00 | 99.02 |
| Length of Sampled Feature | Feature Reduction Technique | |||||||
|---|---|---|---|---|---|---|---|---|
| PCA | LDA | |||||||
| Classifier | Classifier | |||||||
| Quadratic | KNN | ANN | SVM | Quadratic | KNN | ANN | SVM | |
| 11–15 | 35.41 | 24.80 | 34.47 | 72.30 | 34.39 | 50.92 | 57.17 | 65.17 |
| 16–20 | 35.82 | 20.00 | 33.40 | 73.40 | 35.51 | 53.47 | 62.40 | 68.30 |
| 21–25 | 36.12 | 19.10 | 32.50 | 73.10 | 36.73 | 63.47 | 61.35 | 63.35 |
| 26–30 | 34.30 | 20.30 | 36.00 | 68.50 | 41.40 | 67.96 | 73.69 | 64.71 |
| 31–35 | 34.39 | 21.40 | 35.86 | 71.20 | 40.61 | 71.53 | 73.29 | 64.29 |
| 36–40 | 35.71 | 18.78 | 36.79 | 70.30 | 35.71 | 87.04 | 90.05 | 68.84 |
| 41–45 | 34.49 | 18.27 | 35.98 | 69.93 | 34.80 | 94.69 | 89.69 | 63.27 |
| 46–50 | 35.92 | 20.92 | 35.10 | 71.77 | 34.20 | 89.59 | 93.57 | 65.90 |
| 51–55 | 36.43 | 22.45 | 33.97 | 72.61 | 24.90 | 71.22 | 58.78 | 67.61 |
| 56–60 | 36.53 | 22.96 | 32.61 | 71.83 | 25.71 | 66.12 | 58.50 | 64.19 |
| 61–65 | 34.18 | 25.41 | 34.29 | 73.79 | 23.98 | 65.10 | 56.70 | 70.00 |
| 66–70 | 32.45 | 25.00 | 37.56 | 72.35 | 23.16 | 67.04 | 56.11 | 74.90 |
| 71–75 | 30.31 | 24.29 | 38.50 | 71.59 | 27.04 | 66.84 | 48.88 | 74.60 |
| 76–80 | 29.39 | 23.16 | 39.03 | 72.34 | 21.12 | 58.98 | 47.26 | 78.21 |
| 81–85 | 28.98 | 21.73 | 42.09 | 71.33 | 26.02 | 61.94 | 46.94 | 87.12 |
| 86–90 | 27.96 | 17.65 | 42.59 | 72.53 | 26.12 | 57.24 | 52.48 | 85.21 |
| 91–95 | 28.37 | 16.33 | 42.75 | 71.19 | 22.96 | 57.86 | 54.14 | 84.89 |
| 96–100 | 28.37 | 13.57 | 42.80 | 72.33 | 22.24 | 55.31 | 54.90 | 87.30 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bahraminejad, B.; Basri, S.; Isa, M.; Hambli, Z. Real-Time Gas Identification by Analyzing the Transient Response of Capillary-Attached Conductive Gas Sensor. Sensors 2010, 10, 5359-5377. https://doi.org/10.3390/s100605359
Bahraminejad B, Basri S, Isa M, Hambli Z. Real-Time Gas Identification by Analyzing the Transient Response of Capillary-Attached Conductive Gas Sensor. Sensors. 2010; 10(6):5359-5377. https://doi.org/10.3390/s100605359
Chicago/Turabian StyleBahraminejad, Behzad, Shahnor Basri, Maryam Isa, and Zarida Hambli. 2010. "Real-Time Gas Identification by Analyzing the Transient Response of Capillary-Attached Conductive Gas Sensor" Sensors 10, no. 6: 5359-5377. https://doi.org/10.3390/s100605359
APA StyleBahraminejad, B., Basri, S., Isa, M., & Hambli, Z. (2010). Real-Time Gas Identification by Analyzing the Transient Response of Capillary-Attached Conductive Gas Sensor. Sensors, 10(6), 5359-5377. https://doi.org/10.3390/s100605359