Development of a Passive Liquid Valve (PLV) Utilizing a Pressure Equilibrium Phenomenon on the Centrifugal Microfluidic Platform
Abstract
:1. Introduction
2. Materials and Methods
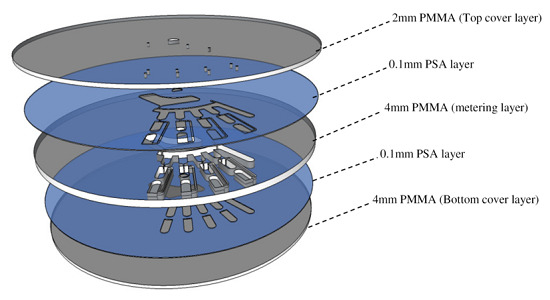
2.1. Design and Fabrication

2.2. Operation Mechanism


| Parameters | Values | Parameters | Values | Parameters | Values |
|---|---|---|---|---|---|
| Ɵc | 68° | rs1 | 32.3 mm | rv2 | 60 mm |
| γla | 71.97 mN/m | rs2 | 37.5 mm | ρ | 1.000 g/cm3 |
| Dh | 3.83e−4 m | rv1 | 58.66, 58.32, 58, 57.65, 56.98 and 56.65 mm |
2.3. Experimental Setup
3. Microfluidic Applications Using PLV

4. Results and Discussion
4.1. Effect of the Liquid Height on the Burst Frequency

4.2. Effect of Liquid Density on the Burst Frequency
4.3. Effect of the Venting Chamber Position on the Burst Frequency
4.4. Liquid Switching and Liquid Metering Results


4.5. Bradford Assay for Measuring Protein Concentrations

5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgment
Author Contributions
Conflicts of Interest
References
- Madou, M.; Zoval, J.; Jia, G.; Kido, H.; Kim, J.; Kim, N. Lab on a CD. Annu. Rev. Biomed. Eng. 2006, 8, 601–628. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.W.; Ahn, C.H. A review of microvalves. J. Micromech. Microeng. 2006, 16. [Google Scholar] [CrossRef]
- Haeberle, S.; Mark, D.; von Stetten, F.; Zengerle, R. Microfluidic Platforms for Lab-On-A-Chip Applications. In Microsystems and Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 853–895. [Google Scholar]
- Yusoff, N.A.; Soin, N.; Ibrahim, F. Lab-on-a-disk as a Potential Microfluidic Platform for Dengue NS1-ELISA. In Proceedings of IEEE Symposium on Industrial Electronics & Applications (ISIEA 2009), Kuala Lumpur, Malaysia, 4–6 October 2009; pp. 946–950.
- Lai, S.; Wang, S.; Luo, J.; Lee, L.J.; Yang, S.-T.; Madou, M.J. Design of a Compact Disk-Like Microfluidic Platform for Enzyme-Linked Immunosorbent Assay. Anal. Chem. 2004, 76, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Jahanshahi, P.; Abd Rahman, N.; Kahar, M.K.B.A.; Madou, M.; Nozari, A.A.; Soin, N.; Dawal, S.Z.M.; Abi Samra, K. Analysis and experiment of centrifugal force for microfluidic ELISA CD platform. In Proceedings of 2010 IEEE EMBS Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 30 November–2 December 2010; pp. 466–470.
- Aeinehvand, M.M.; Ibrahim, F.; Harun, S.W.; Djordjevic, I.; Hosseini, S.; Rothan, H.A.; Yusof, R.; Madou, M. Biosensing Enhancement of dengue virus using microballoon Mixers on centrifugal microfluidic Platforms. Biosens. Bioelectron. 2014. [Google Scholar] [CrossRef]
- Amasia, M.; Cozzens, M.; Madou, M.J. Centrifugal microfluidic platform for rapid PCR amplification using integrated thermoelectric heating and ice-valving. Sens. Actuators B Chem. 2012, 161, 1191–1197. [Google Scholar] [CrossRef]
- Focke, M.; Stumpf, F.; Faltin, B.; Reith, P.; Bamarni, D.; Wadle, S.; Müller, C.; Reinecke, H.; Schrenzel, J.; Francois, P. Microstructuring of polymer films for sensitive genotyping by real-time PCR on a centrifugal microfluidic platform. Lab Chip 2010, 10, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.; Reith, P.; Kijanka, G.; Akujobi, V.; Abgrall, P.; Ducrée, J. Array-based capture, distribution, counting and multiplexed assaying of beads on a centrifugal microfluidic platform. Lab Chip 2012, 12, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Imaad, S.M.; Lord, N.; Kulsharova, G.; Liu, G.L. Microparticle and cell counting with digital microfluidic compact disc using standard CD drive. Lab Chip 2011, 11, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Morijiri, T.; Hikida, T.; Yamada, M.; Seki, M. Microfluidic counterflow centrifugal elutriation for cell separation using density-gradient media. In Proceedings of 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Groningen, The Netherlands, 3–7 October 2010; pp. 722–724.
- Ducrée, J.; Haeberle, S.; Lutz, S.; Pausch, S.; Von Stetten, F.; Zengerle, R. The centrifugal microfluidic Bio-Disk platform. J. Micromech. Microeng. 2007, 17. [Google Scholar] [CrossRef]
- Thio, T.; Nozari, A.A.; Soin, N.; Kahar, M.K.B.A.; Dawal, S.Z.M.; Samra, K.A.; Madou, M.; Ibrahim, F. Hybrid Capillary-Flap Valve for Vapor Control in Point-of-Care Microfluidic CD. In Peoceedings of 5th Kuala Lumpur International Conference on Biomedical Engineering, Kuala Lumpur, Malaysia, 20–23 June 2011; pp. 578–581.
- Siegrist, J.; Gorkin, R.; Clime, L.; Roy, E.; Peytavi, R.g.; Kido, H.; Bergeron, M.; Veres, T.; Madou, M. Serial siphon valving for centrifugal microfluidic platforms. Microfluid. Nanofluid. 2010, 9, 55–63. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Truong, T.-Q. A fully polymeric micropump with piezoelectric actuator. Sens. Actuators B Chem. 2004, 97, 137–143. [Google Scholar] [CrossRef]
- Li, B.; Chen, Q.; Lee, D.-G.; Woolman, J.; Carman, G.P. Development of large flow rate, robust, passive micro check valves for compact piezoelectrically actuated pumps. Sens. Actuators A Phys. 2005, 117, 325–330. [Google Scholar] [CrossRef]
- Kazemzadeh, A.; Ganesan, P.; Ibrahim, F.; Aeinehvand, M.M.; Kulinsky, L.; Madou, M.J. Gating vales on spinning microfluidic platforms: A flow switch/control concept. Sens. Actuators B Chem. 2014, 204, 149–158. [Google Scholar] [CrossRef]
- Al-Faqheri, W.; Ibrahim, F.; Thio, T.H.G.; Moebius, J.; Joseph, K.; Arof, H.; Madou, M. Vacuum/Compression Valving (VCV) Using Parrafin-Wax on a Centrifugal Microfluidic CD Platform. PLOS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abi-Samra, K.; Hanson, R.; Madou, M.; Gorkin III, R.A. Infrared controlled waxes for liquid handling and storage on a CD-microfluidic platform. Lab Chip 2011, 11, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.W.; Namkoong, K.; Chinsung, P. A phase change microvalve using a meltable magnetic material: Ferro-wax. Micro Total Anal. Syst. 2005, 1, 554–556. [Google Scholar]
- Garcia-Cordero, J.L.; Kurzbuch, D.; Benito-Lopez, F.; Diamond, D.; Lee, L.P.; Ricco, A.J. Optically addressable single-use microfluidic valves by laser printer lithography. Lab Chip 2010, 10, 2680–2687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szilágyi, A.; Sumaru, K.; Hattori, K.; Takagi, T.; Filipcsei, G.; Zrínyi, M.; Kanamori, T. On-demand microfluidic control by micropatterned light irradiation of a photoresponsive hydrogel sheet. Lab Chip 2009, 9, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Beebe, D.J. A bi-polymer micro one-way valve. Sens. Actuators A Phys. 2007, 136, 426–433. [Google Scholar] [CrossRef]
- Yang, B.; Lin, Q. A latchable microvalve using phase change of paraffin wax. Sens. Actuators A Phys. 2007, 134, 194–200. [Google Scholar] [CrossRef]
- Thio, T.H.G.; Soroori, S.; Ibrahim, F.; Al-Faqheri, W.; Soin, N.; Kulinsky, L.; Madou, M. Theoretical development and critical analysis of burst frequency equations for passive valves on centrifugal microfluidic platforms. Med. Biol. Eng. Comput. 2013, 51, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Soroori, S.; Kulinsky, L.; Kido, H.; Madou, M. Design and implementation of fluidic micro-pulleys for flow control on centrifugal microfluidic platforms. Microfluid. Nanofluid. 2014, 16, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Metz, T.; Haeberle, S.; Lutz, S.; Ducrée, J.; Zengerle, R.; von Stetten, F. Centrifugo-pneumatic valve for metering of highly wetting liquids on centrifugal microfluidic platforms. Lab Chip 2009, 9, 3599–3603. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Grumann, M.; Geipel, A.; Riegger, L.; Zengerle, R.; Ducrée, J. Batch-mode mixing on centrifugal microfluidic platforms. Lab Chip 2005, 5, 560–565. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Faqheri, W.; Ibrahim, F.; Thio, T.H.G.; Bahari, N.; Arof, H.; Rothan, H.A.; Yusof, R.; Madou, M. Development of a Passive Liquid Valve (PLV) Utilizing a Pressure Equilibrium Phenomenon on the Centrifugal Microfluidic Platform. Sensors 2015, 15, 4658-4676. https://doi.org/10.3390/s150304658
Al-Faqheri W, Ibrahim F, Thio THG, Bahari N, Arof H, Rothan HA, Yusof R, Madou M. Development of a Passive Liquid Valve (PLV) Utilizing a Pressure Equilibrium Phenomenon on the Centrifugal Microfluidic Platform. Sensors. 2015; 15(3):4658-4676. https://doi.org/10.3390/s150304658
Chicago/Turabian StyleAl-Faqheri, Wisam, Fatimah Ibrahim, Tzer Hwai Gilbert Thio, Norulain Bahari, Hamzah Arof, Hussin A. Rothan, Rohana Yusof, and Marc Madou. 2015. "Development of a Passive Liquid Valve (PLV) Utilizing a Pressure Equilibrium Phenomenon on the Centrifugal Microfluidic Platform" Sensors 15, no. 3: 4658-4676. https://doi.org/10.3390/s150304658
APA StyleAl-Faqheri, W., Ibrahim, F., Thio, T. H. G., Bahari, N., Arof, H., Rothan, H. A., Yusof, R., & Madou, M. (2015). Development of a Passive Liquid Valve (PLV) Utilizing a Pressure Equilibrium Phenomenon on the Centrifugal Microfluidic Platform. Sensors, 15(3), 4658-4676. https://doi.org/10.3390/s150304658