Ag-Modified In2O3/ZnO Nanobundles with High Formaldehyde Gas-Sensing Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of In2O3/ZnO Bundles
2.2. Synthesis of Ag-modified In2O3/ZnO Bundles
2.3. Characterization
2.4. HCHO-Sensing Measurement
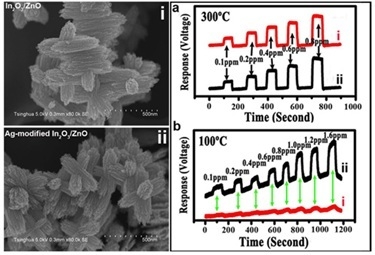
3. Results and Discussion





4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Occupational Safety and Health Administration of Department of Labor. Available online: www.osha.gov/SLTC/formaldehyde/index.html (accessed on 12 January 2014).
- Li, Y.S.; Xu, J.; Chao, J.F.; Chen, D.; Ouyang, S.; Ye, J.H.; Shen, G.Z. High-aspect-ratio single-crystalline porous In2O3 nanobelts with enhanced gas sensing properties. J. Mater. Chem. 2011, 21, 12852–12857. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.J.; Cui, J.B.; Chen, L.P.; Jiang, T.F.; Lin, Y.H. Study on formaldehyde gas-sensing of In2O3-sensitized ZnO nanoflowers under visible light irradiation at room temperature. J. Mater. Chem. 2012, 22, 12915–12920. [Google Scholar] [CrossRef]
- Du, N.; Zhang, H.; Chen, B.D.; Ma, X.Y.; Liu, Z.H.; Wu, J.B.; Yang, D.R. Porous indium oxide nanotubes: Layer-by-layer assembly on carbon-nanotube templates and application for room-temperature NH3 gas sensors. Adv. Mater. 2007, 19, 1641–1645. [Google Scholar] [CrossRef]
- Li, Z.P.; Fan, Y.J.; Zhan, J.H. In2O3 nanofibers and nanoribbons: Preparation by electrospinning and their formaldehyde gas-sensing properties. Eur. J. Inorg. Chem. 2010, 21, 3348–3353. [Google Scholar] [CrossRef]
- Lai, X.Y.; Wang, D.; Han, N.; Du, J.; Li, J.; Xing, C.J.; Chen, Y.F.; Li, X.T. Ordered arrays of bead-Chain-like In2O3 nanorods and their enhanced sensing performance for formaldehyde. Chem. Mater. 2010, 22, 3033–3042. [Google Scholar] [CrossRef]
- Yang, H.X.; Wang, S.P.; Yang, Y.Z. Zn-doped In2O3 nanostructures: Preparation, structure and gas-sensing properties. Cryst. Eng. Comm. 2012, 14, 1135–1142. [Google Scholar] [CrossRef]
- Bai, L.; Fang, F.; Sun, H.Y.; Yan, X.X.; Sun, X.M.; Luo, J.; Zhu, J. Hierarchical ultrathin rolled-up Co(OH)(CO3)0.5 films assembled on Ni0.25Co0.75Sx nanosheets for enhanced supercapacitive performance. RSC Adv. 2014, 4, 57458–57462. [Google Scholar]
- Arandiyan, H.; Dai, H.X.; Deng, J.G.; Wang, Y.; Sun, H.Y.; Xie, S.H.; Bai, B.Y.; Liu, Y.X.; Ji, K.M.; Li, J.H. Three-dimensionally ordered macroporous La0.6Sr0.4MnO3 supported Ag nanoparticles for the combustion of methane. J. Phys. Chem. C. 2014, 118, 14913–14928. [Google Scholar]
- Ahmad, M.; Shi, Y.Y.; Sun, H.Y.; Shen, W.C.; Zhu, J. SnO2/ZnO composite structure for the lithium-ion battery electrode. RSC Adv. 2012, 2, 7901–7905. [Google Scholar]
- Qian, L.H.; Yan, X.Q.; Fujita, T.; Inoue, A.; Chen, M.W. Surface enhanced Raman scattering of nanoporous gold: Smaller pore sizes stronger enhancements. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Chen, L.Y.; Guo, H.; Fujita, T.; Hirata, A.; Zhang, W.; Inoue, A.; Chen, M.W. Nanoporous PdNi bimetallic catalyst with enhanced electrocatalytic performances for electro-oxidation and oxygen reduction reactions. Adv. Funct. Mater. 2011, 21, 4364–4370. [Google Scholar] [CrossRef]
- Fujita, T.; Guan, P.; McKenna, K.; Lang, X.; Hirata, A.; Zhang, L.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Tanaka, N.; et al. Atomic origins of the high catalytic activity of nanoporous gold. Nat. Mater. 2012, 11, 775–780. [Google Scholar] [PubMed]
- Xie, L.M.; Wang, H.L.; Jin, C.H.; Wang, X.R.; Jiao, L.Y.; Suenaga, K.; Dai, H.J. Graphene nanoribbons from unzipped carbon nanotubes: Atomic structures, Raman Spectroscopy, and electrical properties. J. Am. Chem. Soc. 2011, 133, 10394–10397. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Wang, H.L.; Xie, L.M.; Liang, Y.Y.; Hong, G.S.; Dai, H.J. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.G.; Sculley, J.P.; Yuan, D.Q.; Krishna, R.; Wei, Z.W.; Zhou, H.C. Polyamine-tethered porous polymer networks for carbon dioxide capture from flue gas. Angew. Chem. Int. Ed. 2012, 51, 7480–7484. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.J.; Yuan, D.Q.; Li, J.R.; Luo, Z.P.; Zhou, H.C.; Bashir, S.; Liu, J.B. Highly potent bactericidal activity of porous metal-organic frameworks. Adv. Healthc. Mater. 2012, 1, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.Q.; Lu, W.G.; Zhao, D.; Zhou, H.C. Highly stable porous polymer networks with exceptionally high gas-uptake capacities. Adv. Mater. 2011, 23, 3723–3725. [Google Scholar] [CrossRef] [PubMed]
- Gholamia, M.; Khodadadia, A.A.; Firoozb, A.A.; Mortazavic, Y. In2O3-ZnO nanocomposites: High sensor response and selectivity to ethanol. Sens. Actuators B 2015, 212, 395–403. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, G.B.; Lei, X.D.; Luo, L.; Sun, X.M. Mesoporous assembled SnO2 nanospheres: Controlled synthesis, structural analysis and ethanol sensing investigation. Sens. Actuators B 2013, 181, 629–636. [Google Scholar] [CrossRef]
- Singh, V.N.; Mehta, B.R.; Joshi, R.K.; Kruis, F.E.; Shivaprasad, S.M. Enhanced gas sensing properties of In2O3: Ag composite nanoparticle layers: electronic interaction, size and surface induced effects. Sens. Actuators B 2007, 125, 482–488. [Google Scholar] [CrossRef]
- Wang, S.M.; Xiao, B.X.; Yang, T.Y.; Wang, P.; Xiao, C.H.; Li, Z.F.; Zhao, R.; Zhang, M.Z. Enhanced HCHO gas sensing properties by Ag loaded sunflower-like In2O3 hierarchical nanostructures. J. Mater. Chem. A 2014, 2, 6598–6604. [Google Scholar] [CrossRef]
- Zang, W.L.; Nie, Y.X.; Zhu, D.; Deng, P.; Xing, L.L.; Xue, X.Y. Core–shell In2O3/ZnO nanoarray nanogenerator as a self-powered active gas sensor with high H2S sensitivity and selectivity at room temperature. J. Phys. Chem. C 2014, 118, 9209–9216. [Google Scholar] [CrossRef]
- Fu, Y.M.; Zang, W.L.; Wang, P.L.; Xing, L.L.; Xue, X.Y.; Zhang, Y. Portable room-temperature self-powered/active H2 sensor driven by human motion through piezoelectric screening effect. Nano Energy 2014, 8, 34–43. [Google Scholar] [CrossRef]
- Fang, F.; Bai, L.; Sun, H.Y.; Kuang, Y.; Sun, X.M.; Shi, T.; Song, D.S.; Guo, P.; Yang, H.P.; Zhang, Z.F.; et al. Hierarchically porous indium oxide nanolamellas with ten-parts-per-billion-level formaldehyde-sensing performance. Sens. Actuators B 2015, 206, 714–720. [Google Scholar]
- Zhang, L.; Zhao, J.; Lu, H.; Gong, L.; Li, L.; Zheng, J.; Li, H.; Zhu, Z. High sensitive and selective formaldehyde sensors based on nanoparticle-assembled ZnO micro-octahedrons synthesized by homogeneous precipitation method. Sens. Actuators B 2011, 160, 364–370. [Google Scholar] [CrossRef]
- Peng, L.; Zhai, J.; Wang, D.; Zhang, Y.; Wang, P.; Zhao, Q.; Xie, T. Size- and photoelec-tric characteristics-dependent formaldehyde sensitivity of ZnO irradiated with UV light. Sens. Actuators B 2010, 148, 66–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, Q.; Xu, J.Q. High performance chemical sensors constructed by noble metal nanoparticles decorated ZnO nanowires. Sens. Lett. 2011, 9, 332–337. [Google Scholar] [CrossRef]
- Du, H.; Wang, J.; Su, M.; Yao, P.; Zheng, Y.; Yu, N. Formaldehyde gas sensor based on SnO2/In2O3 hetero-nanofibers by a modified double jets electrospinning process. Sens. Actuators B 2012, 166–167, 746–752. [Google Scholar] [CrossRef]
- Castro-Hurtado, I.; Herrán, J.; Mandayo, G.G.; Castaño, E. SnO2-nanowires grown by catalytic oxidation of tin sputtered thin films for formaldehyde detection. Thin Solid Films 2012, 520, 4792–4796. [Google Scholar] [CrossRef]
- Mu, H.C.; Wang, K.K.; Zhang, Z.Q.; Xie, H.F. Formaldehyde graphene gas sensors modified by thermally evaporated tin oxides and tin compound films. J. Phys. Chem. C 2015, 119, 10102–10108. [Google Scholar] [CrossRef]
- Iizuka, K.; Kambara, M.; Yoshida, T. Highly sensitive SnO2 porous film gas sensors fabricated by plasma spray physical vapor deposition. Sens. Actuators, B 2012, 173, 455–461. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, F.; Bai, L.; Song, D.; Yang, H.; Sun, X.; Sun, H.; Zhu, J. Ag-Modified In2O3/ZnO Nanobundles with High Formaldehyde Gas-Sensing Performance. Sensors 2015, 15, 20086-20096. https://doi.org/10.3390/s150820086
Fang F, Bai L, Song D, Yang H, Sun X, Sun H, Zhu J. Ag-Modified In2O3/ZnO Nanobundles with High Formaldehyde Gas-Sensing Performance. Sensors. 2015; 15(8):20086-20096. https://doi.org/10.3390/s150820086
Chicago/Turabian StyleFang, Fang, Lu Bai, Dongsheng Song, Hongping Yang, Xiaoming Sun, Hongyu Sun, and Jing Zhu. 2015. "Ag-Modified In2O3/ZnO Nanobundles with High Formaldehyde Gas-Sensing Performance" Sensors 15, no. 8: 20086-20096. https://doi.org/10.3390/s150820086
APA StyleFang, F., Bai, L., Song, D., Yang, H., Sun, X., Sun, H., & Zhu, J. (2015). Ag-Modified In2O3/ZnO Nanobundles with High Formaldehyde Gas-Sensing Performance. Sensors, 15(8), 20086-20096. https://doi.org/10.3390/s150820086