2.1. Fluorescent Probes for Biothiols
Biothiols, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH), play key roles in physiological systems. It is known that abnormal intracellular thiols are closely related to various health problems. Accordingly, fluorescent probes for these biothiols have attracted great attention in recent years [
21].
A few years ago, our group introduced fluorescein-based probe
1 as a fluorescent probe for biological thiols (
Figure 1) [
22]. As shown in
Figure 1, the spiro lactone ring opening occurred upon the addition of biothiols (GSH, Cys and Hcy) to the α,β-unsaturated ketone, resulting in fluorescence enhancement (λ
max = 520 nm) in HEPES buffer (20 mM, pH 7.4, 1% CH
3CN). To monitor thiols in living cells and organisms, murine P19 embryonic carcinoma cells and a three-day-old zebrafish were incubated with
1. Strong fluorescence enhancement was observed inside the cells and zebrafish. When zebrafish and cells were pretreated with a trapping reagent of thiols,
N-methylmaleimide (NMM), significant fluorescence quenching was observed, which indicates that probe
1 images biothiols, especially GSH, present in the cells or zebra fish.
Figure 1.
Reaction scheme for biothiol selective probe 1.
Figure 1.
Reaction scheme for biothiol selective probe 1.
Non-selective biothiol probes can usually image GSH, the most abundant biothiol in cells. For Cys and Hcy, selective recognition of these biothiols is certainly required, which is quite a challenging task due to the structural similarity of Cys and Hcy. In 2011, a pioneering work was reported by Strongin’s group, in which an α,β-unsaturated carbonyl recognition unit was first utilized to discriminate Cys from Hcy with a kinetically favored 7-membered ring formation [
23].
We recently applied Strongin’s strategy to a new near-infrared (NIR) cyanine-based probe
3 (CyAC), which can selectively sense Cys over Hcy and GSH (
Figure 2) [
24]. In this study, we proposed a new strategy to induce distinct optical changes in the cyanine system. As shown in
Figure 2, the absorption and emission maximums of hydroxy cyanine CyAE
2 and its keto form
2′ can be distinctively different with the modulation of polymethine π-electron systems. Accordingly, the title probe
3 containing an acrylate group as a trigger moiety for Cys can show similar large shifts in its absorption and emission spectra (from 770 to 515 nm for absorption and from 780 to 570 nm for emission) after adduct-cyclization reaction with Cys. Probe
3 was applied to the biological imaging of Cys in MCF-7 cells grown in glucose-free Dulbecco’s modified Eagle medium. During glucose deprivation, the intracellular Cys level is known to be significantly increased. Large fluorescence enhancement at 590 nm and a dramatic fluorescence decrease in the NIR region (760–855 nm) was observed under this condition.
Figure 2.
Comparison between CyAE (2) and CyAK (2′) and reaction scheme for Cys selective probe 3 (CyAC).
Figure 2.
Comparison between CyAE (2) and CyAK (2′) and reaction scheme for Cys selective probe 3 (CyAC).
On the other hand, new pyrene-based fluorescence probes,
4 (
P-Hcy-1) and
5 (
P-Hcy-2), were reported as Hcy selective probes by our group (
Figure 3) [
25].
P-Hcy-1 and
P-Hcy-2 displayed selective fluorescence enhancements at 450 nm with Hcy in HEPES containing 10% DMSO (0.01 M, pH 7.4). In both cases, the thiazinane heterocyclic ring formation of the aldehyde group turned out to be the reason for their Hcy selective fluorescence responses. The detection limits of
P-Hcy-1 and
P-Hcy-2 were calculated as 1.94 × 10
−6 M and 1.44 × 10
−7 M, respectively. These Hcy probes were successfully applied to detect Hcy selectively in mammalian cells.
Among the various approaches to design biothiol selective probes, fluorescence enhancement of Cu
2+ complexes has been utilized for biothiol imaging. A new bis-pyrene derivative
6 was synthesized and a large fluorescence quenching effect was observed with Cu
2+ at pH 7.4 among the various metal ions (
Figure 3) [
26]. The addition of biothiols successfully revived the fluorescence of the
6-Cu
2+ ensemble, which was attributed to the displacement of the ligand to the biothiols for Cu
2+. The addition of GSH, Cys or Hcy effectively induced fluorescence enhancement at 450 nm. The detection limit of this
6-Cu
2+ ensemble for GSH was reported to be 0.16 µM. The Cu
2+ ensemble could successfully image endogenous GSH in live cells and with the aid of two-photon microscopy (TPM), the
6-Cu
2+ ensemble could also image GSH in living tissues.
Figure 3.
Structures of biothiol probes 4–6.
Figure 3.
Structures of biothiol probes 4–6.
Compared to Cys and Hcy selective probes, GSH selective fluorescent imaging probes are still relatively rare. Recently, a new approach to designing GSH selective probes was reported by our group. We synthesized two cyanine derivatives containing a 2,4-dinitrobenzene sulfonamide group (
7) and a 5-dimethylaminonaphthyl sulfonamide group (
8), respectively, as NIR probes for biothiols (
Figure 4) [
27]. Probe
7 showed large fluorescence enhancements at 736 nm with GSH, Cys and Hcy in HEPES buffer (10 mM, pH = 7.4) containing 10% DMSO. On the contrary, probe
8 displayed selective turn-on fluorescence with GSH. After reaction with biothiols, product
9 was confirmed by NMR and mass spectroscopy. These fluorescence changes with biothiols were further confirmed in HeLa cells. Probe
8 could image the GSH present in HeLa cells and no fluorescence was observed upon treatment with the thiol blocking agent,
N-methylmaleimide (NMM). We also demonstrated that probe
9 can be used to monitor the intracellular levels of GSH modulated by H
2O
2 or lipopolysaccharide (LPS) treatment. NIR probe
8 was further applied to monitor GSH in a mouse model (
Figure 5), especially in liver, kidney, lung, and spleen tissues. It is reported that overdose of the painkiller, acetaminophen, can cause severe liver damage and a depletion of GSH in liver and kidney cells. The depletion of GSH in mouse tissue cells was successfully monitored using probe
8.
For Cys and Hcy selective probes, we developed the aryl-thioether substituted nitrobenzothiadiazole
10 (
Figure 6) [
28]. Only Cys and Hcy induced fluorescence enhancement (λ
max = 535 nm) at pH 7.4. The proposed reaction scheme with Cys and Hcy is illustrated in
Figure 6. We also reported that probe
10 could image these biothiol species in live cells. It is known that the nucleophilicity of Cys (
pKa 8.53) is better than that of Hcy (
pKa 10.00). In addition, we expect that the
p-amino group in probe
10 can manipulate the aryl substitution selectivity at lower pH. Indeed, we observed nice selectivity for Cys over Hcy in a citric acid-Na
2HPO
4 (0.01 M, pH 6.0) solution containing 1% DMSO.
Figure 4.
Reaction scheme for biothiol selective probe 7 and GSH selective probe 8.
Figure 4.
Reaction scheme for biothiol selective probe 7 and GSH selective probe 8.
Figure 5.
In vivo images of a mouse injected with probe
8 (50 µM) or NMM (20 mM) intravenously for 20 min. Fluorescence images of: (
A) the mouse not injected with probe
8 (No injection); (
B) the mouse injected with NMM (NMM only); (
C) the mouse injected with probe
8 (
8 only); (
D) the mouse injected with probe
8 after pre-injection with NMM. (Reprinted from reference [
27]).
Figure 5.
In vivo images of a mouse injected with probe
8 (50 µM) or NMM (20 mM) intravenously for 20 min. Fluorescence images of: (
A) the mouse not injected with probe
8 (No injection); (
B) the mouse injected with NMM (NMM only); (
C) the mouse injected with probe
8 (
8 only); (
D) the mouse injected with probe
8 after pre-injection with NMM. (Reprinted from reference [
27]).
Figure 6.
Structure of probe 10 and its reaction scheme with Cys and Hcy.
Figure 6.
Structure of probe 10 and its reaction scheme with Cys and Hcy.
2.2. Fluorescent Imaging Probes for Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS)
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are active targets for fluorescent probes due to their significance in human health and disease [
29]. We also contributed to the development of ROS and RNS selective fluorescent probes, especially for hypochlorous acid (HOCl), hydrogen peroxide (H
2O
2) and peroxynitrite (ONOO
−).
Novel rhodamine derivatives
11–
13 were synthesized as fluorescent probes for HOCl (
Figure 7) [
30]. Fluorescent increase upon the addition of HOCl was attributed to the ring-opening process through sulfur/selenium oxidation as shown in
Figure 7. Probes
11 and
12 showed highly selective fluorescence enhancement with HOCl among the various ROS, such as H
2O
2, NO•, •OH, ROO•, ONOO
−,
1O
2, and •O
2− in KH
2PO
4 buffer (pH 5.5, 1% CH
3CN). On the other hand, probe
13 displayed a lower selectivity for HOCl, probably due to the higher susceptibility to selenolactone by oxidants.The detection limits of HOCl probes
11–
13 were calculated as 0.4 μM, 0.6 μM and 2 μM, respectively. Furthermore, probe
11 could image bacteria-mediated microbicidal HOCl production in the mucosal epithelia in fruit fly.
Figure 7.
Structures of HOCl selective fluorescent probes 11–13 and reaction scheme for 11 with HOCl.
Figure 7.
Structures of HOCl selective fluorescent probes 11–13 and reaction scheme for 11 with HOCl.
We extended this design concept to the so-called “dual-lock” fluorescent probe
14 (
FBS) for HOCl (
Figure 8) [
31], in which two reacting groups, such as aryl boronate and thiolactone, are introduced. Among the various ROS, H
2O
2 and ONOO
− could convert arylboronates to phenol to yield
14′, which is still non-fluorescent. On the contrary, only HOCl can react with both arylboronates and thiolactone to give fluorescein (
14″) resulting in green fluorescence. Another advantage of
FBS would be its large pH window between pH 5.5 and pH 9.3.
FBS was further applied to detect physiological HOCl production
in vivo. For this purpose, we chose the
drosophila gut system, a well-known HOCl producing organ. FBS could successfully image bacterial-induced HOCl production
in situ when bacterial extracts were administered to the flies via oral ingestion.
Figure 8.
Reaction scheme for HOCl selective fluorescent probe 14 (FBS).
Figure 8.
Reaction scheme for HOCl selective fluorescent probe 14 (FBS).
Recently, the imidazoline-2-thione containing OCl
− probes,
15 (
PIS) and
16 (
NIS), were designed as new fluorescent probes for HOCl (
Figure 9) [
32]. Upon the addition of up to 5 μM OCl
−, a new absorbance peak for
PIS at 378 nm appeared with the sacrifice of the peak at 420 nm in PBS (pH 7.4). Addition of OCl
− (0–10 µM) also induced a new fluorescence emissionat 505 nm. We believe the PIS reaction with HOCl generates imidazolium salt
17 and the proposed mechanism is illustrated in
Figure 9.
NIS displayed similar changes with shorter emission wavelengths. To demostrate the possible bio-applications of these probes,
PIS was used to visualize OCl
− generation in RAW 264.7 macrophages, which were activated by lipopolysaccharides (LPS) and then IFN-γ. H
2O
2 produced by phorbol myristate acetate (PMA) was transformed to OCl
− by MPO. As expected, bright green fluorescence was observed in RAW 264.7 macrophages. When the known MPO inhibitors, 4-aminobenzoicacid hydrazide (ABAH) and flufenamic acid (FFA) were added, distinct fluorescence quenching was observed, which means that
PIS successfully visualized OCl
− production in RAW 264.7 macrophages. We designed a co-culture system of RAW 264.7 macrophages and HeLa cell. When these cell mixtures were treated with stimulants to generate OCl
−, the macrophages and HeLa cells had distinguishably different shapes and further, green fluorescence was observed only for RAW 264.7 macrophages. Finally,
PIS was utilized to detect OCl
− by using TPM. As shown in
Figure 10, under similar conditions of RAW 264.7 macrophage experiments,
PIS could successfully image OCl
− production.
Figure 9.
Structures of 15 (PIS) and 16 (NIS) and reaction scheme of 15 (PIS) with HOCl.
Figure 9.
Structures of 15 (PIS) and 16 (NIS) and reaction scheme of 15 (PIS) with HOCl.
Figure 10.
TPM images of (
a–
e)
PIS and (
f)
16 (10 μM, ρ
DMF = 0.5%) labeled RAW 264.7 cells. (
a) Control image; (
b) Cells pretreated with NaOCl (200 μM) for 30 min and then incubated with
PIS; (
c) Cells pretreated with LPS (100 ng/mL) for 16 h, IFN-γ (400 U/mL) for 4 h, and PMA (10 nM) for 30 min and then with
PIS; (
d) Cells pretreated with LPS, IFN-γ, and 4-ABAH (50 μM) for 4 h and then incubated with
PIS; (
e) Cells pretreated with LPS, IFN-γ, and FAA (50 μM) for 4 h and then with
PIS; (
g) Average TPEF intensities in (
a–
f),
n = 5. Scale bar: 20 μm. (Reprinted from reference [
32]).
Figure 10.
TPM images of (
a–
e)
PIS and (
f)
16 (10 μM, ρ
DMF = 0.5%) labeled RAW 264.7 cells. (
a) Control image; (
b) Cells pretreated with NaOCl (200 μM) for 30 min and then incubated with
PIS; (
c) Cells pretreated with LPS (100 ng/mL) for 16 h, IFN-γ (400 U/mL) for 4 h, and PMA (10 nM) for 30 min and then with
PIS; (
d) Cells pretreated with LPS, IFN-γ, and 4-ABAH (50 μM) for 4 h and then incubated with
PIS; (
e) Cells pretreated with LPS, IFN-γ, and FAA (50 μM) for 4 h and then with
PIS; (
g) Average TPEF intensities in (
a–
f),
n = 5. Scale bar: 20 μm. (Reprinted from reference [
32]).
James and our group recently reported a unique system for sensing peroxynitrite (ONOO
−) using boronate-based fluorescent probe
17 and
d-fructose (
Figure 11) [
33]. When
d-fructose was added to probe
17 at pH 7.3, a large fluorescence enhancement was observed. Among the various ROS and RNS species, only ONOO
− induced a significant fluorescence quenching effect. We believe that the unique interaction of probe
17 with
d-fructose plays two important roles in this study, namely to strengthen the fluorescence signal and to prevent the oxidation of boronic acid by other ROS/RNS. This system was also successfully applied to image endogenous and exogenous ONOO
− in RAW 264.7 cells and HeLa cells.
Figure 11.
Reaction scheme for probe 17 with ONOO−.
Figure 11.
Reaction scheme for probe 17 with ONOO−.
The red emitting probe
18 (
CHCN) composed of a linked coumarin-hemicyanine was recently developed as a dual ratiometric and colorimetric probe for peroxynitrite (ONOO
−) (
Figure 12) [
34]. Among the various ROS and RNS, only ONOO
− induced significant ratiometric fluorescence changes (F
515nm/F
635nm). The proposed reaction mechanism is shown in
Figure 12. The formation of two products, 1,3,3-trimethyloxindole and Coum-CHO was also confirmed. As shown in
Figure 13,
18 (
CHCN) displayed ratiometric fluorescence changes for exogenous and endogenous ONOO
− during the phagocytic immune response. More specifically, red fluorescence was reduced with the enhancement of green fluorescence when RAW 264.7 cells were treated with LPS and IFN-g followed by additional stimulation with PMA. Treatment with a superoxide scavenger, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) or an NO synthase inhibitor, aminoguanidine, caused no changes in the ratios of red/green fluorescence.
Recently, a new boronate-based naphthalimide derivative
19 was developed as a H
2O
2 selective fluorescent probe (
Figure 14) [
35].
Figure 14 explains the design strategy for probe
19, in which a morpholine moiety acts as a lysosome-targetable group and a
p-dihydroxyborylbenzyloxycarbonyl group was used as a transponder. Among various ROS and RNS, probe
19 showed a selective fluorescent enhancement at 528 nm at pH 7.4 (0.1 M PBS containing 1% DMF). Probe
19 was clearly localized in the lysosomes, which was confirmed by costaining with LysoTracker Blue DND-22. Probe
19 was further applied to image the level of endogenous and exogenous H
2O
2 in the lysosome of the RAW 264.7 cells. Furthermore, time-dependent fluorescence of
19 in the cells was reported.
Figure 12.
Reaction scheme of peroxinitrite probe 18.
Figure 12.
Reaction scheme of peroxinitrite probe 18.
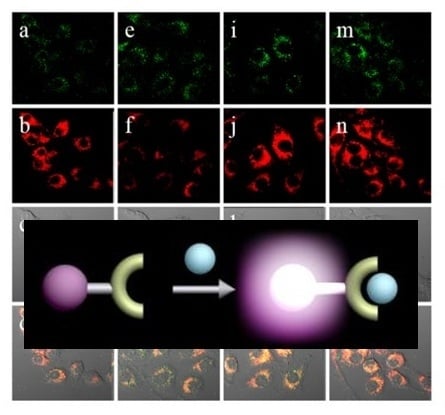
Figure 13.
Confocal ratiometric fluorescence images of RAW 264.7 cells for endogenous ONOO
− during phagocytic immune response. The cells were stained with 5 μM
18 (
CHCN) for 30 min and then washed with DPBS before imaging. (
a) Control; (
e) lipopolysaccharides (LPS) (1 μg/mL) for 16 h, interferon-γ (50 ng/mL) for 4 h, PMA (10 nM) for 30 min; (
i) LPS (1 μg/mL) for 16 h, interferon-γ (50 ng/mL) for 4 h, PMA (10 nM) for 30 min, and then AG (1 mM) for 16 h; (
m) LPS (1 μg/mL) or 16 h, interferon-γ (50 ng/mL) for 4 h, PMA (10 nM) for 30 min, and then TEMPO (100 μM) for 16 h. The green channel (
a,
e,
I,
m) represents the fluorescence obtained at 490–540 nm with an excitation wavelength at 473 nm, the red channel (
b,
f,
j,
n) represents the fluorescence obtained at 575–675 nm with an excitation wavelength at 559 nm, images (
c,
g,
k,
o) represent DIC channels (differential interference contrast), and images (
d,
h,
I,
p) represent merged images of red and green channels, respectively. (Reprinted from reference [
34]).
Figure 13.
Confocal ratiometric fluorescence images of RAW 264.7 cells for endogenous ONOO
− during phagocytic immune response. The cells were stained with 5 μM
18 (
CHCN) for 30 min and then washed with DPBS before imaging. (
a) Control; (
e) lipopolysaccharides (LPS) (1 μg/mL) for 16 h, interferon-γ (50 ng/mL) for 4 h, PMA (10 nM) for 30 min; (
i) LPS (1 μg/mL) for 16 h, interferon-γ (50 ng/mL) for 4 h, PMA (10 nM) for 30 min, and then AG (1 mM) for 16 h; (
m) LPS (1 μg/mL) or 16 h, interferon-γ (50 ng/mL) for 4 h, PMA (10 nM) for 30 min, and then TEMPO (100 μM) for 16 h. The green channel (
a,
e,
I,
m) represents the fluorescence obtained at 490–540 nm with an excitation wavelength at 473 nm, the red channel (
b,
f,
j,
n) represents the fluorescence obtained at 575–675 nm with an excitation wavelength at 559 nm, images (
c,
g,
k,
o) represent DIC channels (differential interference contrast), and images (
d,
h,
I,
p) represent merged images of red and green channels, respectively. (Reprinted from reference [
34]).
![Sensors 15 24374 g013]()
Figure 14.
Design strategy of H2O2 selective probe 19 and its reaction scheme with H2O2.
Figure 14.
Design strategy of H2O2 selective probe 19 and its reaction scheme with H2O2.
2.3. Fluorescent Imaging Probes for Metal Ions
Among the biologically abundant metal ions, Zn
2+ is known to play key roles in a variety of physiological processes, such as Alzheimer’s disease, epilepsy, ischemic stroke,
etc. Due to the lower reactivity of this metal ion, most Zn
2+ selective fluorescent probes are based on the design of selective ligands and effective fluorescence changes [
36].
In 2009, we reported a 7-nitrobenz-2-oxa-1,3-diazole (NBD) derivative as a fluorescent and colorimetric probe for Zn
2+ (
Figure 15) [
37]. Among the various metal ions, probe
20 displayed a selective fluorescence enhancement only with Zn
2+ in 100% aqueous solution (0.1 M HEPES, pH 7.2). In addition, red to yellow color change with Zn
2+ was observed by the naked eye. A large fluorescence enhancement as well as colorimetric changes was attributed to photoinduced electron transfer (PET) and internal charge transfer (ICT) mechanisms as shown in
Figure 15. The dissociation constant (
Kd) for
20 was reported as 1.3 μM. Relatively high concentrations of Zn
2+ are present in pancreatic islets, which are known to play a key role in insulin biosynthesis and storage. The practical-use probe
20 could successfully detect the intrinsic Zn
2+ Ions in pancreatic β-cells.
Figure 15.
Proposed binding mode of probe 20 with Zn2+.
Figure 15.
Proposed binding mode of probe 20 with Zn2+.
We recently proposed a new strategy called “receptor transformer” [
38]. As shown in
Figure 16, the naphthalimide derivative (
21) binds Zn
2+ in an imidic acid tautomeric form of the probe in aqueous solutions while other heavy transition metal ions, especially Cd
2+, bind to probe
21 in an amide tautomeric form. With the aid of these unique binding modes, probe
21 displayed a selective fluorescence enhancement for Zn
2+ over other metal ions with a red-shift from 483 to 514 nm. On the other hand, the addition of Cd
2+ induced an enhanced blue-shift in emission from 483 to 446 nm in aqueous solution, which binds to probe
21 with an amide tautomeric form. Therefore, green and blue fluorescence was observed for
in vitro and
in vivo Zn
2+ and Cd
2+, respectively. These different binding modes were confirmed by NMR and IR data. The dissociation constants (
Kd) for Zn
2+ and Cd
2+ were reported as 5.7 nM and 48.5 nM, respectively. Finally, as shown in
Figure 17,
21 could successfully detect intrinsic zinc ions during the development of living zebrafish embryos.
Figure 16.
Proposed binding modes of probe 21 with metal ions.
Figure 16.
Proposed binding modes of probe 21 with metal ions.
Figure 17.
Zebrafish incubated with probe
21 (5 μM). (
a) Images of 19 h-old; (
b) 36 h-old; and (
c) 48 h-old zebrafish incubated with
21 for 1 h; (
d) Image of 54 h-old zebrafish incubated with
21 for 1 h and (
e) image of 54 h-old zebrafish after initial incubation with 100 μM TPEN for 1 h and subsequent treatment of washed zebrafish with
21 for 1 h. (Reprinted from reference [
38]).
Figure 17.
Zebrafish incubated with probe
21 (5 μM). (
a) Images of 19 h-old; (
b) 36 h-old; and (
c) 48 h-old zebrafish incubated with
21 for 1 h; (
d) Image of 54 h-old zebrafish incubated with
21 for 1 h and (
e) image of 54 h-old zebrafish after initial incubation with 100 μM TPEN for 1 h and subsequent treatment of washed zebrafish with
21 for 1 h. (Reprinted from reference [
38]).
A new cyanine-based NIR fluorescent probe
22 bearing tris(2-pyridylmethyl)amine (TMPA) was reported by our group to detect endogenous zinc ions in living cells and organisms (
Figure 18) [
39,
40]. The unique hypsochromic shifts with Zn
2+ in absorption (670–510 nm) and emission (730–590 nm) were explained as follows: Introduction of Zn
2+ to the TMPA moiety in
22 deprotonated the amine attached to the center of the polymethine chain of tricarbocyanine to form an imine, which induced the less delocalized diamino-tetraene group. Probe
22 displayed a very strong binding affinity (
Kd = 1.2 nM), which means that probe
22 can serve as an excellent probe to monitor Zn
2+ in the nanomolar range. Most importantly, probe
22 was used to detect Zn
2+ released during apoptosis induced by the addition of H
2O
2. It is known that metallothioneins, expressed in neuromasts, play a key role in Zn
2+ homeostasis [
41].
Figure 19 clearly shows that probe
22 could detect intact Zn
2+ in the neuromasts of zebrafish.
Figure 18.
Proposed binding mode of probe 22 with Zn2+.
Figure 18.
Proposed binding mode of probe 22 with Zn2+.
Figure 19.
Fluorescent detection of intact zinc ions in zebra fish using probe
22 (
a) 24 h; (
b) 36 h; (
c) 48 h; (
d) 72 h and (
e) 96 h-old zebra fish incubated with probe
22 for 1 h. (Reprinted from reference [
39]).
Figure 19.
Fluorescent detection of intact zinc ions in zebra fish using probe
22 (
a) 24 h; (
b) 36 h; (
c) 48 h; (
d) 72 h and (
e) 96 h-old zebra fish incubated with probe
22 for 1 h. (Reprinted from reference [
39]).
Due to the high toxicity of mercury and methyl mercury, the development of new fluorescent probes has been actively explored by many groups [
42]. During the last few years, our group has utilized the unique ring-opening process of spirolactam in rhodamine derivatives to develop fluorescent and colorimetric probes for various metal ions in which the ring-opening process of spirolactam can induce large fluorescent enhancements and colorimetric changes (colorless to red) [
43,
44].
Two simple rhodamine hydrazone derivatives bearing thiol (
23) and carboxylic acid (
24) groups were developed as selective fluorescent and colorimetric probes for Hg
2+ (
Figure 20) [
45]. Upon the addition of Hg
2+, the selective ring-opening process of spirolactams in probes
23 and
24 were observed in CH
3CN-H
2O (1:99,
v/
v) solution, which was accompanied by large fluorescent enhancement and colorimetric change. The detection limits were calculated as 1 nM for
23 and 4.2 nM for
24. Both probes could successfully image Hg
2+ accumulated in the nematode
C. elegans, which was pretreated with nanomolar concentrations of Hg
2+.
Figure 20.
Structures of Hg2+ selective probes 23–25.
Figure 20.
Structures of Hg2+ selective probes 23–25.
On the other hand, a rhodamine-based sensor
25 bearing a histidine group was also reported as a selective probe for Hg
2+ (
Figure 20) [
46]. Upon the addition of 100 equiv Hg
2+ in 0.02 M pH 7.4 HEPES: EtOH (1:9,
v/
v), turn-on fluorescence (100-fold) was observed due to the spiro-lactam ring opening. We believe that imidazole nitrogen and two carbonyl oxygenscan provided a nice binding site for Hg
2+. Probe
25 was further applied to visualize Hg
2+ in HeLa cells.
A rhodamine derivative bearing the selenolactone group
26 was synthesized for the detection of inorganic and organic mercury species (
Figure 21) [
47]. Through the mercury ion-promoted deselenation reaction, probe
26 displayed highly selective fluorescent and colorimetric changes for mercury species with high sensitivity in 20 mM HEPES buffer (pH 7.4, 1% CH
3CN). We then attempted to monitor the mercury species that accumulated in living organisms using
26. Zebrafish has been used as a suitable animal model for the study of fluorescent probes [
48]. For our study, adult zebrafish was pre-incubated with Hg
2+ (500 nM) or methylmercury (500 nM) and then with probe
26. As shown in
Figure 22, strong red fluorescence was observed in the gallbladder, eggs and fin due to the presence of inorganic mercury and methylmercury.
Figure 21.
Proposed reaction scheme for probe 26 with Hg2+ and CH3Hg+.
Figure 21.
Proposed reaction scheme for probe 26 with Hg2+ and CH3Hg+.
Figure 22.
Images of zebrafish organs treated with 20 µM
26 (0.2% DMSO) and 500 nM HgCl
2 or 500 nM CH
3HgCl. (
a) Images of zebrafish organs treated with
26 in the absence of HgCl
2 or CH
3HgCl; (
b) presence of 500 nM HgCl
2 or (
c) presence of 500 nM CH
3HgCl (upper, merged images; lower, fluorescence images). (Reprinted from reference [
47]).
Figure 22.
Images of zebrafish organs treated with 20 µM
26 (0.2% DMSO) and 500 nM HgCl
2 or 500 nM CH
3HgCl. (
a) Images of zebrafish organs treated with
26 in the absence of HgCl
2 or CH
3HgCl; (
b) presence of 500 nM HgCl
2 or (
c) presence of 500 nM CH
3HgCl (upper, merged images; lower, fluorescence images). (Reprinted from reference [
47]).
Rhodamine derivative bearing boronic acid (
27) was studied as a probe for Cu
2+ for the first time (
Figure 23) [
49]. When Cu
2+ was introduced to probe
27 in 20 mM HEPES (0.5% CH
3CN) at pH 7.4, selective turn-on fluorescence and distinct colorimetric change was observed, which was also attributed to the Cu
2+-induced spirolactam ring opening process. Probe
27 was applied to visualize Cu
2+ in cells and zebra fish.
Figure 23.
Proposed binding mode of probe 27 with Cu2+.
Figure 23.
Proposed binding mode of probe 27 with Cu2+.
A reaction based fluorescent probe based on rhodamine-alkyne derivative
28 was synthesized for the detection of Au
3+ (
Figure 24) [
50]. The addition of Au
3+ induced a highly selective turn-on fluorescence and colorimetric change in EtOH-HEPES buffer (0.01 M, pH 7.4) (1:1,
v/
v). The proposed reaction mechanism from the propargylamide moiety of
28 to the oxazolecarbaldehyde of
29 is illustrated in
Figure 24. The rate constant for the conversion of
28 to
29 was reported to be
Kobs = 4.5 (±0.20) × 10
−4 s
−1 and the detection limit was calculated as 320 nM. In addition, probe
28 was successfully applied to detect Au
3+ in the cell.
Figure 24.
Proposed reaction scheme of probe 28 with Au3+.
Figure 24.
Proposed reaction scheme of probe 28 with Au3+.
A 4-Amino-1,8-naphthalimide derivative bearing an alkyne group (
30) was explored as a fluorescent probe for Au
3+ (
Figure 25 and
Figure 26) [
51]. The addition of Au
3+ induced a distinct color change from yellow to light pink and a large blue shift by about 56 nm in the emission spectra. The detection limit was calculated to be 8.44 μM. We observed that surfactants could enhance the reaction rate of probe
30 with Au
3+. Probe
30 was also applied to detect Au
3+ in HeLa cells and differentiated adipocytes. In particular, probe
30 showed a unique ability to detect Au
3+ in lipid droplets in cells. As shown in
Figure 26, the response rates of
30 with Au
3+ in differentiated adipocytes were greater than those in HeLa cells, which is consistent with the rate enhancement in the presence of surfactants.
Figure 25.
Structure of the probe 30.
Figure 25.
Structure of the probe 30.
Figure 26.
(
a) Response rates of
30 to Au
3+ in HeLa cells and differentiated adipocytes. HeLa cells and adipocytes were incubated with 100 μM AuCl
3 for 2 h and then with
30 (20 μM) for 2, 4, 6, and 8 h (■: HeLa cells, ▲: adipocytes); (
b) Time-dependent fluorescence images of HeLa cells and adipocytes treated with
30 and Au
3+ (scale bar = 20 μm). (Reprinted from reference [
51]).
Figure 26.
(
a) Response rates of
30 to Au
3+ in HeLa cells and differentiated adipocytes. HeLa cells and adipocytes were incubated with 100 μM AuCl
3 for 2 h and then with
30 (20 μM) for 2, 4, 6, and 8 h (■: HeLa cells, ▲: adipocytes); (
b) Time-dependent fluorescence images of HeLa cells and adipocytes treated with
30 and Au
3+ (scale bar = 20 μm). (Reprinted from reference [
51]).
2.4. Fluorescent Imaging Probes for Cyanide and ATP
Cyanide ion is a toxic anion. Especially, HCN production by
Pseudomonas aeruginosa (PA) is known to be closely related to the pathogenesis of CF lung disease. Accordingly, the detection of cyanide in
in vivo systems has become an important issue for bacterial cyanogenesis and CF patients. Cyanide selective fluorescent probes have been actively studied using various design strategies [
52].
To develop a new cyanide imaging probe, we firstly synthesized NIR fiuorescent probe sensor
31 (
Figure 27) [
53]. When Cu
2+ was added and the complex
31-Cu2+ was formed, the absorption maximum shifted from 718 to 743 nm and fluorescence quenching (748 nm) was observed in 20 mM HEPES buffer (pH 7.4, 0.5% CH
3CN). Upon the addition of CN
− to
31-Cu2+, fluorescence emission at 748 nm was recovered since CN
− and Cu
2+ form the stable [Cu(CN)
x]
n−. The detection limit for CN
− was reported as 5 μM. Probe
31-Cu2+ was applied to visualize the CN
− produced by
P. aeruginosa (PA) in
C. elegans cells. To confirm the bacterial infection in the intestine of the nematodes, a PA14 strain labeled with GFP (green fluorescent protein) was introduced for the
C. elegans to feed on before the imaging. As shown
Figure 28a, neither green nor NIR fluorescence was observed in the nematodes after
C. elegans exposure to only
E. coli OP50 and probe
31-Cu2+. On the contrary, when nematodes were exposed to PA14 and the probe, both green and NIR fluorescence were observed, which indicates that the probe can image HCN produced by PA14 in the nematodes (
Figure 28b–d). As shown in
Figure 29, treatment with a β-lactam antibiotic, ceftazidime, significantly reduced both the green and NIR fluorescence intensities.
Figure 27.
Structure of cyanide selective probe 31 and 31-Cu2+ complex.
Figure 27.
Structure of cyanide selective probe 31 and 31-Cu2+ complex.
Figure 28.
NIR imaging of cyanide in
C. elegans infected with a
P. aeruginosa strain (PA14) labeled with green fluorescent protein (GFP). Before the imaging, the nematodes fed on either non-infectious
E. coli OP50 (
a) or GFP-labeled PA14 for 2 days (
b–
d); (
b) the anterior end; (
c) the medial part; (
d) the posterior end of
C. elegans. The scale bars represent 20 μm. (IL = intestinal lumen; I = intestine; E = eggs; PA = PA14-GFP; A = anus). (Reprinted from reference [
54]).
Figure 28.
NIR imaging of cyanide in
C. elegans infected with a
P. aeruginosa strain (PA14) labeled with green fluorescent protein (GFP). Before the imaging, the nematodes fed on either non-infectious
E. coli OP50 (
a) or GFP-labeled PA14 for 2 days (
b–
d); (
b) the anterior end; (
c) the medial part; (
d) the posterior end of
C. elegans. The scale bars represent 20 μm. (IL = intestinal lumen; I = intestine; E = eggs; PA = PA14-GFP; A = anus). (Reprinted from reference [
54]).
Imidazolium derivatives serve as host molecules for the recognition of various anions since they can form a unique (C–H)
+—anion type ionic hydrogen bond [
54,
55,
56].
We synthesized an imidazolium receptor bearing two pyrene groups (
32) as ATP selective probes, in which a pyrene excimer acts as a signal source and imidazoliums serve as the triphosphate anion receptor (
Figure 30) [
57]. Among the various nucleoside triphosphates, only ATP showed significant ratiometric changes in its emission. More specifically, only ATP induced an increase in monomer emission at 375 with a sacrifice of excimer emission at 487 nm. We believe this result can be attributed to the different binding mode of ATP compared to the other four nucleoside triphosphate bases: GTP, CTP, UTP and TTP. As shown in
Figure 30, ATP can form a characteristic sandwich π-π stack of pyrene-adenine-pyrene while GTP, CTP, UTP and TTP interact with probe
32 from the outside, with the stacked pyrene-pyrene dimer of
32 resulting in only excimer fluorescence quenching. These different binding modes were proposed based on NMR study and theoretical calculation results. Oligomycin is reported to decrease cellular ATP levels. Probe
32 showed strong blue fluorescence in HeLa cells and the fluorescence was quenched upon the addition of oligomycin.
Figure 29.
Visualization of antibiotic efficacy against
P. aeruginosa infection in
C. elegans with the NIR sensor. The nematodes fed on GFP-labeled
P. aerugionosa (PA14) for two days. They were then incubated with ceftazidime (200 μg/mL) for 2 h before the
in vivo imaging. The scale bars represent 20 μm. (Reprinted from reference [
53]).
Figure 29.
Visualization of antibiotic efficacy against
P. aeruginosa infection in
C. elegans with the NIR sensor. The nematodes fed on GFP-labeled
P. aerugionosa (PA14) for two days. They were then incubated with ceftazidime (200 μg/mL) for 2 h before the
in vivo imaging. The scale bars represent 20 μm. (Reprinted from reference [
53]).
Figure 30.
Proposed binding modes of probe 32 with ATP and GTP.
Figure 30.
Proposed binding modes of probe 32 with ATP and GTP.