Optimizing Colorimetric Assay Based on V2O5 Nanozymes for Sensitive Detection of H2O2 and Glucose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of V2O5 Nanozymes
2.3. Physical Characterization
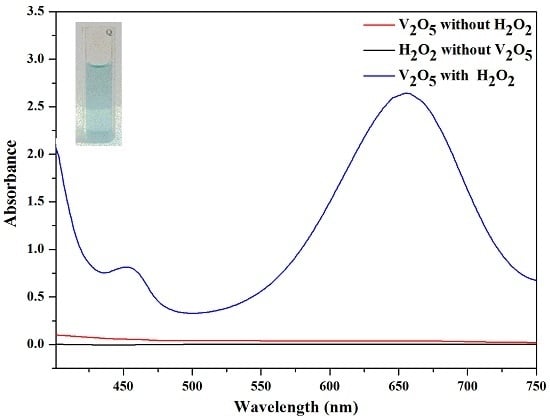
2.4. H2O2 Detection Using V2O5 Nanozymes
2.5. Glucose Detection Using V2O5 Nanozymes
3. Results and Discussion
3.1. Characterization of V2O5 Nanozymes
3.2. Principle
3.3. Effect of pH
3.4. Effect of Buffers
3.5. Effect of V2O5 Nanozymes Concentrations
3.6. Calibration Curve for H2O2 Detection
3.7. Calibration Curve for Glucose Detection
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GOx | glucose oxidase |
| HRP | horseradish peroxidase |
| OPD | o-phenylenediamine |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| LOD | limit of detection |
| Vmax | maximum initial velocity |
| Km | Michaelis-Menten constant |
References
- Wei, H.; Wang, E.K. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.Z.; Zhuang, J.; Nie, L.; Zhang, J.B.; Zhang, Y.; Gu, N.; Wang, T.H.; Feng, J.; Yang, D.; Perrete, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.M. Iron oxide nanoparticles: Hidden talent. Nat. Nanotechnol. 2007, 2, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Comotti, M.; Pina, C.D.; Matarrese, R.; Rossi, M. The Catalytic Activity of “Naked” Gold Particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.S.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.S.; Wang, Y.; Zhao, M.; Zhang, L. Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem. Commun. 2012, 48, 2540–2542. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.F.; Cao, H.Q.; Lu, Y.X. Self-assembly into magnetic Co3O4 complex nanostructures as peroxidase. J. Mater. Chem. 2012, 22, 527–534. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J.; Feng, Y.B.; Hong, L.; Chen, Q.Y.; Wu, L.F.; Lin, X.H.; Xia, X.H. Peroxidase-like activity of water-soluble cupric oxide nanoparticles and its analytical application for detection of hydrogen peroxide and glucose. Analyst 2012, 137, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Qi, P.; Zhang, D.; Wu, J.J.; Wang, Y. Manganese oxide nanowire-mediated enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2012, 33, 69–74. [Google Scholar] [CrossRef] [PubMed]
- André, R.; Natálio, F.; Humanes, M.; Leppin, J.; Heinze, K.; Wever, R.; Schröder, H.-C.; Müller, W.E.G.; Tremel, W. V2O5 Nanowires with an Intrinsic Peroxidase-Like Activity. Adv. Funct. Mater. 2011, 21, 501–509. [Google Scholar] [CrossRef]
- Shi, W.B.; Wang, Q.L.; Long, Y.J.; Cheng, Z.L.; Chen, S.H.; Zheng, H.Z.; Huang, Y.M. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 7, 6695–6697. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Qu, K.G.; Zhao, C.; Ren, J.S.; Qu, X.G. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- Jv, Y.; Li, B.X.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Ju, E.G.; Ren, J.S.; Qu, X.G. Polypyrrole nanoparticles as promising enzyme mimics for sensitive hydrogen peroxide detection. Chem. Commun. 2014, 50, 3030–3032. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.R.; Cairelli, S.G.; Whalen, J.J. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs); National Institute for Occupational Safety and Health: Washington, DC, USA, 1994. [Google Scholar]
- Nagaraja, P.; Shivakumar, A.; Shrestha, A.K. Quantification of hydrogen peroxide and glucose using 3-methyl-2-benzothiazolinonehydrazone hydrochloride with 10,11-dihydro-5H-benz(b,f)azepine as chromogenic probe. Anal. Biochem. 2009, 395, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Tang, H.Q. Optical determination of glucose and hydrogen peroxide using a nanocomposite prepared from glucose oxidase and magnetite nanoparticles immobilized on graphene oxide. Microchim. Acta. 2014, 181, 527–534. [Google Scholar] [CrossRef]
- Guilbault, G.G.; Brignac, P.J.; Zimmer, M. Homovanillic acid as a fluorometric substrate for oxidative enzymes. Analytical applications of the peroxidase, glucose oxidase, and xanthine oxidase systems. Anal. Chem. 1968, 40, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Li, B.X.; Zhang, Z.J. Chemiluminescence flow biosensor for glucose based on gold nano particle-enhanced activities of glucose oxidase and horseradish peroxidase. Biosens. Bioelectron. 2008, 24, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Zhu, L.H.; Jiang, G.D.; Tang, H.Q. Sensitive fluorescent probes for determination of hydrogen peroxide and glucose based on enzyme-immobilized magnetite/silica nanoparticles. Anal. Bioanal. Chem. 2009, 395, 2277–2385. [Google Scholar] [CrossRef] [PubMed]
- Fink, D.; Klinkovich, I.; Bukelman, O.; Marks, R.S.; Kiv, A.; Fuks, D.; Fahrner, W.R.; Alfonta, L. Glucose determination using a re-usable enzyme-modified ion track membrane sensor. Biosens. Bioelectron. 2009, 24, 2702–2706. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.M.; Arakawa, H.; Yamada, M. Flow injection chemiluminescent determination of trace amounts of hydrogen peroxide in snow-water using KIO4–K2CO3 system. Anal. Chim. Acta 1998, 371, 171–176. [Google Scholar] [CrossRef]
- Li, Y.Z.; Townshend, A. Evaluation of the adsorptive immobilisation of horseradish peroxidase on PTFE tubing in flow systems for hydrogen peroxide determination using fluorescence detection. Anal. Chim. Acta 1998, 359, 149–156. [Google Scholar] [CrossRef]
- Xu, J.R.; Chen, Z.M. Determination of Peroxides in Environmental Samples by High Performance Liquid Chromatography. Chin. J. Chromatogr. 2005, 23, 366–369. [Google Scholar]
- Wang, C.H.; Yang, C.; Song, Y.Y.; Gao, W.; Xia, X.H. Adsorption and Direct Electron Transfer from Hemoglobin into a Three-Dimensionally Ordered Macroporous Gold Film. Adv. Funct. Mater. 2005, 15, 1267–1275. [Google Scholar] [CrossRef]
- Tarpey, M.M.; Wink, D.A.; Grisham, M.B. Methods for detection of reactive metabolites of oxygen and nitrogen: In vitro and in vivo considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R431–R444. [Google Scholar] [CrossRef] [PubMed]
- Ozalp, V.C.; Zeydanli, U.S.; Lunding, A.; Kavruk, M.; Oz, M.T.; Eyidogan, F.; Olsen, L.F.; Oktem, H.A. Nanoparticle embedded enzymes for improved lateral flow sensors. Analyst 2013, 138, 4255–4259. [Google Scholar] [CrossRef] [PubMed]
- Shoji, E.; Freund, M.S. Potentiometric sensors based on the inductive effect on the pK(a) of poly(aniline): A nonenzymatic glucose sensor. J. Am. Chem. Soc. 2001, 123, 3383–3384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhao, X.M.; Yuan, C.G.; Li, L. Vanadium Pentoxide Nanowires: Hydrothermal Synthesis, Formation Mechanism, and Phase Control Parameters. Cryst. Growth Des. 2008, 8, 723–727. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.Q.; Wang, L.; Zhang, Y.W.; Luo, Y.L.; Li, H.Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Fast and Sensitive Colorimetric Detection of H2O2 and Glucose: A Strategy Based on Polyoxometalate Clusters. Chempluschem 2012, 77, 541–544. [Google Scholar] [CrossRef]
- Wang, J.J.; Han, D.X.; Wang, X.H.; Qi, B.; Zhao, M.S. Polyoxometalates as peroxidase mimetics and their applications in H2O2 and glucose detection. Biosens. Bioelectron. 2012, 36, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.I.; Shim, J.; Li, T.; Lee, J.; Park, H.G. Fabrication of Nanoporous Nanocomposites Entrapping Fe3O4 Magnetic Nanoparticles and Oxidases for Colorimetric Biosensing. Chem. Eur. J. 2011, 17, 10700–10707. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, X.D.; Hu, J. Pt-DNA complexes as peroxidase mimetics and their applications in colorimetric detection of H2O2 and glucose. Anal. Methods 2012, 4, 2183–2187. [Google Scholar] [CrossRef]
- Edgar, P.; Yona, K. A Simple Colorimetric Method for the Measurement of Hydrogen Peroxide Produced by Cells in Culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar]








| Nanozymes | Substrate | KM (mM) | Vmax (M·s−1) |
|---|---|---|---|
| V2O5 nanozymes | TMB | 0.738 | 1.85 × 10−5 |
| V2O5 nanozymes | H2O2 | 0.232 | 1.29 × 10−5 |
| Fe3O4 MNPs | TMB | 0.434 | 10.00 × 10−8 |
| Fe3O4 MNPs | H2O2 | 154 | 9.78 × 10−8 |
| HRP | TMB | 0.434 | 1.24 × 10−8 |
| HRP | H2O2 | 3.70 | 2.46 × 10−8 |
| Nanozymes | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|
| H4SiW12O40 | 1–20 μM | 0.4 μM | [31] |
| H3PW12O40 | 0.134–67 μM | 0.134 μM | [32] |
| Fe3O4 MNPs | 1–100 μM | 0.5 μM | [33] |
| Pt-DNA complexes | 0.979–17.6 mM | 0.392 mM | [34] |
| HRP | 1–60 μM | 1 μM | [35] |
| V2O5 nanozymes | 1–500 μM | 1 μM | This work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Li, C.; Qi, Y.; Guo, S.; Liang, X. Optimizing Colorimetric Assay Based on V2O5 Nanozymes for Sensitive Detection of H2O2 and Glucose. Sensors 2016, 16, 584. https://doi.org/10.3390/s16040584
Sun J, Li C, Qi Y, Guo S, Liang X. Optimizing Colorimetric Assay Based on V2O5 Nanozymes for Sensitive Detection of H2O2 and Glucose. Sensors. 2016; 16(4):584. https://doi.org/10.3390/s16040584
Chicago/Turabian StyleSun, Jiaheng, Chunyan Li, Yanfei Qi, Shuanli Guo, and Xue Liang. 2016. "Optimizing Colorimetric Assay Based on V2O5 Nanozymes for Sensitive Detection of H2O2 and Glucose" Sensors 16, no. 4: 584. https://doi.org/10.3390/s16040584
APA StyleSun, J., Li, C., Qi, Y., Guo, S., & Liang, X. (2016). Optimizing Colorimetric Assay Based on V2O5 Nanozymes for Sensitive Detection of H2O2 and Glucose. Sensors, 16(4), 584. https://doi.org/10.3390/s16040584