Single-Use Disposable Electrochemical Label-Free Immunosensor for Detection of Glycated Hemoglobin (HbA1c) Using Differential Pulse Voltammetry (DPV)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus and Reagents
2.2. Electrode Fabrication
2.3. Electrode Functionalization
2.3.1. Pretreatment of Gold Electrode (AuE)
2.3.2. Anti-HbA1c Immobilization on AuE
2.4. Electrochemical Measurements
2.5. X-ray Photoelectron Spectroscopy
3. Results and Discussion
3.1. MPA-SAM Characterization
3.1.1. X-ray Photoelectron Spectroscopy
3.1.2. EIS Assessment of MPA-SAM Surface Coverage
3.2. HbA1c Detection Using Differential Pulse Voltammetry (DPV)
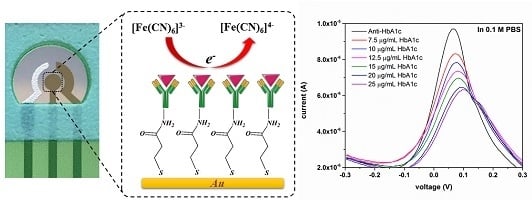
3.2.1. HbA1c Detection in 0.1 M PBS
3.2.2. HbA1c Detection in Undiluted Human Serum
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goldstein, D.E.; Little, R.R.; Lorenz, R.A.; Malone, J.I.; Nathan, D.; Peterson, C.M.; Sacks, D.B. Tests of glycemia in diabetes. Diabetes Care 2004, 27, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, E.S. HbA1c measurement. J. Clin. Pathol. 2004, 57, 344–345. [Google Scholar] [CrossRef] [PubMed]
- John, W.G. Haemoglobin A1c reference method. Scand. J. Clin. Lab. Investig. 2006, 66, 1–4. [Google Scholar] [CrossRef] [PubMed]
- John, W.G. Haemoglobin A1c: Analysis and standardisation. Clin. Chem. Lab. Med. 2003, 41, 1199–1212. [Google Scholar] [CrossRef] [PubMed]
- Jeppsson, J.O. Approved IFCC Reference Method for the Measurement of HbA1c in Human Blood. Clin. Chem. Lab. Med. 2002, 40, 78. [Google Scholar] [CrossRef] [PubMed]
- Eckerbom, S.; Bergqvist, Y.; Jeppsson, J.O. Improved method for analysis of glycated haemoglobin by ion exchange chromatography. Ann. Clin. Biochem. 1994, 31, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Frantzen, F.; Grimsrud, K.; Heggli, D.E.; Faaren, A.L.; Løvli, T.; Sundrehagen, E. Glycohemoglobin filter assay for doctors’ offices based on boronic acid affinity principle. Clin. Chem. 1997, 43, 2390–2396. [Google Scholar] [PubMed]
- Zhao, Z.; Basilio, J.; Hanson, S.; Little, R.R.; Sumner, A.E.; Sacks, D.B. Evaluation of hemoglobin A1c measurement by Capillarys 2 electrophoresis for detection of abnormal glucose tolerance in African immigrants to the United States. Clin. Chim. Acta 2015, 446, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. A novel type of fluorescent boronic acid that shows large fluorescence intensity changes upon binding with a carbohydrate in aqueous solution at physiological pH. Bioorg. Med. Chem. Lett. 2003, 13, 1019–1022. [Google Scholar] [CrossRef]
- Kataoka, K.; Hisamitsu, I.; Sayama, N.; Okano, T.; Sakurai, Y. Novel Sensing System for Glucose Based on the Complex Formation between Phenylborate and Fluorescent Diol Compounds. J. Biochem. 1995, 117, 1145–1147. [Google Scholar] [PubMed]
- Wang, B.; Anzai, J. Recent Progress in Electrochemical HbA1c Sensors: A Review. Materials 2015, 8, 1187–1203. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, H.; Liu, L.; Hao, Y.; Chang, Z.; Xu, M. Fabrication of electrochemical interface based on boronic acid-modified pyrroloquinoline quinine/reduced graphene oxide composites for voltammetric determination of glycated hemoglobin. Biosens. Bioelectron. 2014, 64, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Rawat, S.; Bhalla, V.; Suri, C.R. Point-of-Care Amperometric Testing of Diabetic Marker (HbA1c) Using Specific Electroactive Antibodies. Electroanalysis 2014, 26, 469–472. [Google Scholar] [CrossRef]
- Kim, D.M.; Shim, Y.B. Disposable amperometric glycated hemoglobin sensor for the finger prick blood test. Anal. Chem. 2013, 85, 6536–6543. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Chang, B.-Y.; Nam, H.; Park, S.-M. Selective Electrochemical Sensing of Glycated Hemoglobin (HbA(1c)) on Thiophene-3-Boronic Acid Self-Assembled Monolayer Covered Gold Electrodes. Anal. Chem. 2008, 80, 8035–8044. [Google Scholar] [CrossRef] [PubMed]
- Halámek, J.; Wollenberger, U.; Stöcklein, W.; Scheller, F.W. Development of a biosensor for glycated hemoglobin. Electrochim. Acta 2007, 53, 1127–1133. [Google Scholar] [CrossRef]
- Bhat, N.; Srinivasan, S. Detection of GlycatedHemoglobin using 3-AminoPhenylboronic acid modified Graphene Oxide. In Proceedings of the IEEE/NIH Life Science Systems and Applications Workshop (LiSSA), Bethesda, MD, USA, 7–8 April 2011; pp. 1–4.
- Janyasupab, M.; Lee, Y.; Zhang, Y.; Liu, C.W.; Cai, J.; Popa, A.; Samia, A.C.; Wang, K.W.; Xu, J.; Hu, C.-C.; et al. Detection of Lysyl Oxidase-Like 2 (LOXL2), a Biomarker of Metastasis from Breast Cancers Using Human Blood Samples. Curr. Biomark. 2015, 5, 93–100. [Google Scholar] [CrossRef]
- Willner, I.; Riklin, A.; Wlllner, I.; Rlklln, A. Electrical Communication between Electrodes and NAD(P)+-Dependent Enzymes Using Pyrroloquinolinequinone-Enzyme Electrodes in a Self-Assembled Monolayer Configuration: Design of a New Class of Amperometric Biosensors. Anal. Chem. 1994, 66, 1535–1539. [Google Scholar] [CrossRef]
- Campuzano, S.; Glávez, R.; Pedrero, M.; de Villena, F.J.M.; Pingarrón, J.M. Preparation, characterization and application of alkanethiol self-assembled monolayers modified with tetrathiafulvalene and glucose oxidase at a gold disk electrode. J. Electroanal. Chem. 2002, 526, 92–100. [Google Scholar] [CrossRef]
- Moscovici, M.; Bhimji, A.; Kelley, S.O. Rapid and specific electrochemical detection of prostate cancer cells using an aperture sensor array. Lab Chip 2013, 13, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Glidle, A.; Griffith, A.; McNeil, C.J.; Cooper, J.M. Characterising the formation of a bioelectrochemical interface at a self-assembled monolayer using X-ray photoelectron spectroscopy. Bioelectrochem. Bioenerg. 1997, 42, 15–23. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, L.-R.; Chen, W.; Yang, X.-J.; Jin, B.; Zheng, L.-M.; Xia, X.-H. 3-Mercaptopropylphosphonic Acid Modified Gold Electrode for Electrochemical Detection of Dopamine. Bioelectrochemistry 2009, 75, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Bourg, M.C.; Badia, A.; Lennox, R.B. Gold-sulfur bonding in 2D and 3D self-assembled monolayers: XPS characterization. J. Phys. Chem. B 2000, 104, 6562–6567. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Likhatski, M.; Tomashevich, Y.; Romanchenko, A.; Erenburg, S.; Trubina, S. XAS and XPS examination of the Au-S nanostructures produced via the reduction of aqueous gold(III) by sulfide ions. J. Electron Spectrosc. Relat. Phenom. 2010, 177, 24–29. [Google Scholar] [CrossRef]
- Wu, J.; Campuzano, S.; Halford, C.; Haake, D.A.; Wang, J. Ternary surface monolayers for ultrasensitive (Zeptomole) amperometric detection of nucleic acid hybridization without signal amplification. Anal. Chem. 2010, 82, 8830–8837. [Google Scholar] [CrossRef] [PubMed]
- Janek, R.P.; Fawcett, W.R.; Ulman, A. Impedance Spectroscopy of Self-Assembled Monolayers on Au(111): Sodium Ferrocyanide Charge Transfer at Modified Electrodes. Langmuir 1998, 14, 3011–3018. [Google Scholar] [CrossRef]
- Song, S.Y.; Yoon, H.C. Boronic acid-modified thin film interface for specific binding of glycated hemoglobin (HbA1c) and electrochemical biosensing. Sens. Actuators B Chem. 2009, 140, 233–239. [Google Scholar] [CrossRef]







| Take-off Angle | –COOH Count | O–C=O Count | –COOH/O–C=O Atomic Ratio |
|---|---|---|---|
| 10° | 122.20 | 547.62 | 0.2231 |
| 50° | 330.02 | 1385.55 | 0.2382 |
| 90° | 164.08 | 516.98 | 0.3174 |
| Surface | ||||||||
|---|---|---|---|---|---|---|---|---|
| bare AuE | 1.31 | 972.6 | 367.7 | 201.5 | 107 | |||
| MPA-SAM | 0.46 | 1738 | 74,542 | 10,347 | 152 | 0.9805 | 0.9950 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molazemhosseini, A.; Magagnin, L.; Vena, P.; Liu, C.-C. Single-Use Disposable Electrochemical Label-Free Immunosensor for Detection of Glycated Hemoglobin (HbA1c) Using Differential Pulse Voltammetry (DPV). Sensors 2016, 16, 1024. https://doi.org/10.3390/s16071024
Molazemhosseini A, Magagnin L, Vena P, Liu C-C. Single-Use Disposable Electrochemical Label-Free Immunosensor for Detection of Glycated Hemoglobin (HbA1c) Using Differential Pulse Voltammetry (DPV). Sensors. 2016; 16(7):1024. https://doi.org/10.3390/s16071024
Chicago/Turabian StyleMolazemhosseini, Alireza, Luca Magagnin, Pasquale Vena, and Chung-Chiun Liu. 2016. "Single-Use Disposable Electrochemical Label-Free Immunosensor for Detection of Glycated Hemoglobin (HbA1c) Using Differential Pulse Voltammetry (DPV)" Sensors 16, no. 7: 1024. https://doi.org/10.3390/s16071024
APA StyleMolazemhosseini, A., Magagnin, L., Vena, P., & Liu, C. -C. (2016). Single-Use Disposable Electrochemical Label-Free Immunosensor for Detection of Glycated Hemoglobin (HbA1c) Using Differential Pulse Voltammetry (DPV). Sensors, 16(7), 1024. https://doi.org/10.3390/s16071024