Enhancement of Fluorescence-Based Sandwich Immunoassay Using Multilayered Microplates Modified with Plasma-Polymerized Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Deposition of Metal Layers
2.3. Preparations of PPFs
2.4. Homogeneous Fluorescence Detection of Cy3-Labeled Antibody in the Multilayered Microplate
2.5. Fluorescence-Based Immunoassay Targeting Antigen Directly Adsorbed on the Multilayered Microplate
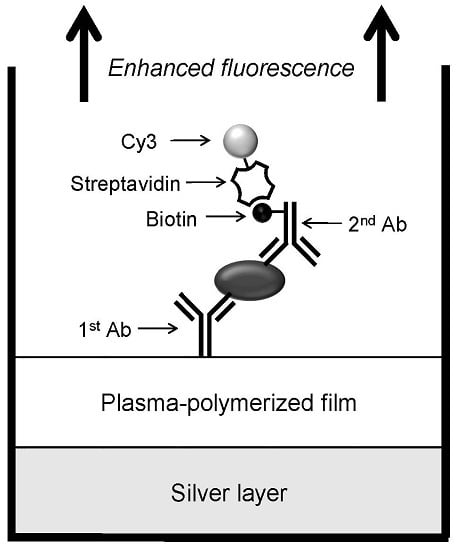
2.6. Sandwich Immunoassay of IL-2 on the Multilayered Microplates
3. Results and Discussion
3.1. Calibration Curves for Thin Layer Deposition on the Surface of Microplates
3.2. Homogeneous Fluorescence Detection of Cy3-Labeled Antibody in the Multilayered Microplate
3.3. Fluorescence-Based Immunoassay Targeting Antigen Directly Adsorbed on the Multilayered Microplate
3.4. Sandwich Immunoassay of IL-2 on the Multilayered Microplates
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sapsford, K.E.; Blanco-Canosa, J.B.; Dawson, P.E.; Medintz, I.L. Detection of HIV-1 specific monoclonal antibodies using enhancement of dye-labeled antigenic peptides. Bioconjugate Chem. 2010, 21, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Sang, C.-H.; Chou, S.-J.; Pan, F.M.; Sheu, J.-T. Fluorescence enhancement and multiple protein detection in ZnO nanostructure microfluidic devices. Biosens. Bioelectron. 2016, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Darvill, D.; Centeno, A.; Xie, F. Plasmonic fluorescence enhancement by metal nanostructures: Shaping the future of bionanotechnology. Phys. Chem. Chem. Phys. 2013, 15, 15709–15726. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Duyne, R.P.V. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Kaya, T.; Takei, H. Characterization of cap-shaped silver particles for surface-enhanced fluorescence effects. Anal. Biochem. 2007, 364, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, M.; Qiang, W.; Hu, H.; Li, W.; Xu, D. Metal-enhanced fluorescent detection for protein microarrays based on a silver plasmonic substrate. Analyst 2014, 139, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Keegan, G.L.; Stranik, O.; Brennan-Fournet, M.E.; McDonagh, C. Highly sensitive C-reactive protein (CRP) assay using metal-enhanced fluorescence (MEF). J. Nanoparticle Res. 2015, 17, 326–337. [Google Scholar] [CrossRef]
- Du, L.; Zhang, C.; Wang, L.; Liu, G.; Zhang, Y.; Wang, S. Ultrasensitive time-resolved microplate fluorescence immunoassay for bisphenol A using a system composed on gold nanoparticles and a europium(III)-labeled streptavidin tracer. Microchim. Acta 2015, 182, 539–545. [Google Scholar] [CrossRef]
- Holland, W.R.; Hall, D.G. Waveguide mode enhancement of molecular fluorescence. Opt. Lett. 1985, 10, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.G.; King, O.; Sigg, C.; Hall, D.G. Directional, enhanced fluorescence from molecules near a periodic surface. Appl. Opt. 1994, 33, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Hiep, H.M.; Yoshikawa, H.; Saito, M.; Tamiya, E. An interference localized surface plasmon resonance biosensor based on the photonic structure of Au nanoparticles and SiO2/Si multilayers. ACS Nano 2009, 3, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Yasuda, M.; Karube, I. Effect of the polarization and incident angle of excitation light on the fluorescence enhancement observed with a multilayered substrate fabricated by Ag and Al2O3. Appl. Opt. 2008, 47, 3789–3794. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Akimoto, T. High-contrast fluorescence imaging based on the polarization dependence of the fluorescence enhancement using an optical interference mirror slide. Anal. Sci. 2015, 31, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Etoh, H.; Yasuda, M.; Akimoto, T. Signal enhancement by a multi-layered substrate for mutagen detection using an SOS response-induced green fluorescent protein in genetically modified Escherichia coli. Anal. Sci. 2011, 27, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Akimoto, T. Highly sensitive fluorescence detection of avidin/streptavidin with an optical interference mirror slide. Anal. Sci. 2012, 28, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Le Moal, E.; Fort, E.; Lévêque-Fort, S.; Cordelières, F.P.; Fontaine-Aupart, M.-P.; Ricolleau, C. Enhanced fluorescence cell imaging with metal-coated slides. Biophys. J. 2007, 92, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, A.; Karube, I. Plasma polymerized films for sensor devices. Electroanalysis 2000, 12, 695–702. [Google Scholar] [CrossRef]
- Muguruma, H. Plasma-polymerized films for biochip design. Plasma Process. Polym. 2010, 7, 151–162. [Google Scholar] [CrossRef]
- Behnisch, J.; Tyczkowski, J.; Gazicki, M.; Pela, I.; Holländer, A.; Ledzion, R. Formation of hydrophobic layers on biologically degradable polymeric foils by plasma polymerization. Surf. Coat. Technol. 1998, 98, 872–874. [Google Scholar] [CrossRef]
- Görbig, O.; Nehlsen, S.; Müller, J. Hydrophobic properties of plasma polymerized thin film gas selective membranes. J. Membrane Sci. 1998, 138, 115–121. [Google Scholar] [CrossRef]
- Tserepi, A.; Gogolides, E.; Bourkoula, A.; Kanioura, A.; Kokkoris, G.; Petrou, P.S.; Kakabakos, S.E. Plasma nanotextured polymeric surfaces for controlling cell attachment and proliferation: A short review. Plasma Chem. Plasma Process. 2016, 36, 107–120. [Google Scholar] [CrossRef]
- Muguruma, H.; Hiratsuka, A.; Karube, I. Thin-film glucose biosensor based on plasma-polymerized film: Simple design for mass production. Anal. Chem. 2000, 72, 2671–2675. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, H.; Hoshino, T.; Matsui, Y. Enzyme biosensor based on plasma-polymerized film-covered carbon nanotube layer grown directly on a flat substrate. ACS Appl. Mater. Interfaces 2011, 3, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Muguruma, H.; Ikebukuro, K.; Sasaki, S.; Nagata, R.; Karube, I.; Pedersen, H. A plasma-polymerized film for surface plasmon resonance immunosensing. Anal. Chem. 1997, 69, 4649–4652. [Google Scholar] [CrossRef]
- Kojima, K.; Hiratsuka, A.; Suzuki, H.; Yano, K.; Ikebukuro, K.; Karube, I. Electrochemical protein chip with arrayed immunosensors with antibodies immobilized in a plasma-polymerized film. Anal. Chem. 2003, 75, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, H.; Ikebukuro, K.; Yano, K.; Aburatani, H.; Karube, I. Single nucleotide polymorphism typing on DNA array with hydrophobic surface fabricated by plasma-polymerization technique. Biosens. Bioelectron. 2004, 20, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Ishiwata, S.; Tsuji, Y.; Dejima, M.; Yano, K.; Takase, I.; Karube, I. Application of probes having 2’-deoxyinosine for typing of single nucleotide polymorphisms (SNPs) using DNA microarray. Anal. Chim. Acta 2006, 561, 25–31. [Google Scholar] [CrossRef]
- Tsai, S.-W.; Loughran, M.; Hiratsuka, A.; Yano, K.; Karube, I. Application of plasma-polymerized films for isoelectric focusing of proteins in a capillary electrophoresis chip. Analyst 2003, 128, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, H.; Hiratsuka, A.; Ikebukuro, K.; Yano, K.; Muguruma, H.; Karube, I. Application of polymer-embedded proteins to fabrication of DNA array. Biotechnol. Bioeng. 2000, 69, 323–329. [Google Scholar] [CrossRef]
- Chu, L.; Knoll, W.; Förch, R. Biologically multifunctional surfaces using plasma polymerization methods. Plasma Process. Polym. 2006, 3, 498–505. [Google Scholar] [CrossRef]
- Tajima, I.; Yamamoto, M. Spectroscopic study on chemical structure of plasma-polymerized hexamethyldisiloxane. J. Polymer Sci. Polym. Chem. Ed. 1985, 23, 615–622. [Google Scholar] [CrossRef]
- Schwarz, J.; Schmidt, M.; Ohl, A. Synthesis of plasma-polymerized hexamethyldisiloxane (HMDSO) films by microwave discharge. Surf. Coat. Technol. 1998, 98, 859–864. [Google Scholar] [CrossRef]
- Yano, K.; Yamano, K.; Iwasaki, A.; Akimoto, T.; Miyachi, H.; Hiratsuka, A. Fluorescence enhancement of immunoassay using multilayered glass substrates modified with plasma-polymerized films. Sens. Mater. 2015, 27, 859–869. [Google Scholar]
- Piletsky, S.A.; Piletska, E.V.; Bossi, A.; Karim, K.; Lowe, P.; Turner, A.P.F. Substitution of antibodies and receptors with molecularly imprinted polymers in enzyme-linked and fluorescent assays. Biosens. Bioelectron. 2001, 16, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Jarrell, J.D.; Eun, T.H.; Samale, M.; Briant, C.; Sheldon, B.W.; Morgan, J.R. Metal oxide coated cell culture arrays for rapid biological screening. J. Biomed. Mater. Res. A 2007, 83, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lee, K.H.; Park, H.; Choi, J. Enhanced detection of single-cell-secreted proteins using a fluorescent immunoassay on the protein-G-terminated glass substrate. Int. J. Nanomed. 2015, 10, 7197–7205. [Google Scholar]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yano, K.; Iwasaki, A. Enhancement of Fluorescence-Based Sandwich Immunoassay Using Multilayered Microplates Modified with Plasma-Polymerized Films. Sensors 2017, 17, 37. https://doi.org/10.3390/s17010037
Yano K, Iwasaki A. Enhancement of Fluorescence-Based Sandwich Immunoassay Using Multilayered Microplates Modified with Plasma-Polymerized Films. Sensors. 2017; 17(1):37. https://doi.org/10.3390/s17010037
Chicago/Turabian StyleYano, Kazuyoshi, and Akira Iwasaki. 2017. "Enhancement of Fluorescence-Based Sandwich Immunoassay Using Multilayered Microplates Modified with Plasma-Polymerized Films" Sensors 17, no. 1: 37. https://doi.org/10.3390/s17010037
APA StyleYano, K., & Iwasaki, A. (2017). Enhancement of Fluorescence-Based Sandwich Immunoassay Using Multilayered Microplates Modified with Plasma-Polymerized Films. Sensors, 17(1), 37. https://doi.org/10.3390/s17010037