A New Laccase Based Biosensor for Tartrazine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
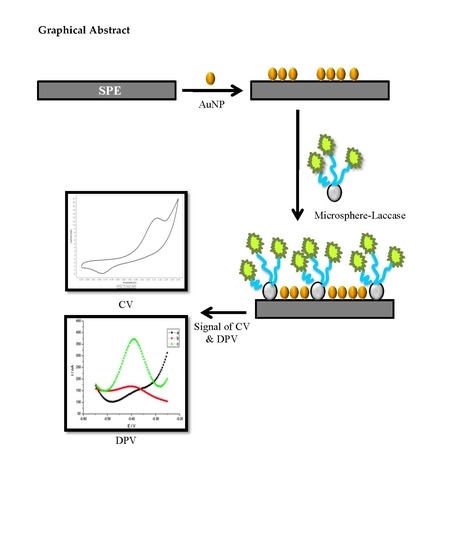
2.2. Fabrication of Functionalized Microspheres and Electrochemical Characterization
2.3. Optimization and Evaluation of Electrochemical Tartrazine Biosensor
2.4. Determination of Tartrazine in Food Samples
2.5. Validation and Recovery Studies of Tartrazine in Food Samples
3. Results
3.1. Methacrylate-Acrylate Microspheres
3.2. Electrochemical Behaviours of Tartrazine
3.3. Dependence of Biosensor Response on pH and Exposure Time
3.4. Effect of Microspheres and Nanogold Loading on Enzyme Biosensor Response
3.5. Influence of Laccase Immobilization Time
3.6. Linear Range and Detection Limit of Tartrazine Biosensor
3.7. Validation of Tartrazine Biosensor in Food Samples
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Minussi, R.C.; Pastore, G.M.; Durán, N. Potential applications of laccase in the food industry. Trends Food Sci. Technol. 2002, 13, 205–216. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J. Facile synthesis of Au supported on ionic liquid functionalized reduced graphene oxide for simultaneous determination of sunset yellow and tartrazine in drinks. Sens. Actuators B Chem. 2015, 216, 578–585. [Google Scholar] [CrossRef]
- Zalacain, A.; Ordoudi, S.; Blazquez, I.; Díaz-Plaza, E.M.; Carmona, M.; Tsimidou, M.; Alonso, G. Screening method for the detection of artificial colours in saffron using derivative UV-Vis spectrometry after precipitation of crocetin. Food Addit. Contam. 2005, 22, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Ichihashi, K. Determination of 40 synthetic food colors in drinks and candies by high-performance liquid chromatography using a short column with photodiode array detection. Talanta 2008, 74, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Zhao, Y.; Yong, W.; Sun, L.; Jiang, G.; Chu, X. Highly sensitive and accurate screening of 40 dyes in soft drinks by liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. B 2011, 879, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-based biosensors for detection of phenolic compounds. TrAC Trends Anal. Chem. 2015, 74, 21–45. [Google Scholar] [CrossRef]
- Ling, Y.P.; Heng, L.Y. A potentiometric formaldehyde biosensor based on immobilization of alcohol oxidase on acryloxysuccinimide-modified acrylic microspheres. Sensors 2010, 10, 9963–9981. [Google Scholar] [CrossRef] [PubMed]
- Hanifah, S.A.; Hamzah, N.; Lee, Y.H. Rapid synthesis of magnetic microspheres poly (Glycidyl Methacrylate-co-Styrene) by photopolymerization. Sains Malays. 2013, 42, 487–493. [Google Scholar]
- Bayramoğlu, G.; Kiralp, S.; Yilmaz, M.; Toppare, L.; Arıca, M.Y. Covalent immobilization of chloroperoxidase onto magnetic beads: Catalytic properties and stability. Biochem. Eng. J. 2008, 38, 180–188. [Google Scholar] [CrossRef]
- Danisman, T.; Tan, S.; Kacar, Y.; Ergene, A. Covalent immobilization of invertase on microporous pHEMA–GMA membrane. Food Chem. 2004, 85, 461–466. [Google Scholar] [CrossRef]
- Brady, D.; Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett. 2009, 31, 1639. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lou, Y.; Wan, Y.; Wang, W.; Yao, J.; Zhang, B. Laccase immobilized onto poly (GMA-MAA) microspheres for p-benzenediol removal from wastewater. Water Sci. Technol. 2013, 67, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Doğan, T.; Bayram, E.; Uzun, L.; Şenel, S.; Denizli, A. Trametes versicolor laccase immobilized poly (glycidyl methacrylate) based cryogels for phenol degradation from aqueous media. J. Appl. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Mao, Y.; Fan, Q.; Li, J.; Yu, L.; Qu, L.-B. A novel and green CTAB-functionalized graphene nanosheets electrochemical sensor for Sudan I determination. Sens. Actuators B Chem. 2014, 203, 759–765. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Cao, S.; Gao, F.; Zhang, Y.; Yang, Y. One-step electrochemical approach for the preparation of graphene wrapped-phosphotungstic acid hybrid and its application for simultaneous determination of sunset yellow and tartrazine. Electrochim. Acta 2012, 74, 151–157. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Shih, Y.-C.; Chen, Y.-C. Determining eight colorants in milk beverages by capillary electrophoresis. J. Chromatogr. A 2002, 959, 317–325. [Google Scholar] [CrossRef]
- Karim-Nezhad, G.; Khorablou, Z.; Zamani, M.; Dorraji, P.S.; Alamgholiloo, M. Voltammetric sensor for tartrazine determination in soft drinks using poly (p-aminobenzenesulfonic acid)/zinc oxide nanoparticles in carbon paste electrode. J. Food Drug Anal. 2017, 25, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, S.Z.; Hanifah, S.A. Synthesis and effect of modification on methacylate-acrylate microspheres for Trametes versicolor laccase enzyme immobilization. AIP Conf. Proc. 2014, 1, 263–268. [Google Scholar]
- Mazlan, S.Z.; Hanifah, S.A. Effects of Temperature and pH on Immobilized Laccase Activity in Conjugated Methacrylate-Acrylate Microspheres. Int. J. Polym. Sci. 2017, 2017, 8. [Google Scholar] [CrossRef]
- Fu, J.; Qiao, H.; Li, D.; Luo, L.; Chen, K.; Wei, Q. Laccase Biosensor Based on Electrospun Copper/Carbon Composite Nanofibers for Catechol Detection. Sensors 2014, 14, 3543–3556. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Guo, M.Q.; Wang, X.J.; Wang, H.F.; Chen, W.Y. Development of Amperometric Laccase Biosensor through Immobilizing Enzyme in Magnesium-Containing Mesoporous Silica Sieve (Mg-MCM-41)/Polyvinyl Alcohol Matrix. J. Nanomater. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Alves, S.P.; Brum, D.M.; de Andrade, É.C.B.; Netto, A.D.P. Determination of synthetic dyes in selected foodstuffs by high performance liquid chromatography with UV-DAD detection. Food Chem. 2008, 107, 489–496. [Google Scholar] [CrossRef]
- Ulianas, A.; Heng, L.Y.; Ahmad, M. A biosensor for urea from succinimide-modified acrylic microspheres based on reflectance transduction. Sensors 2011, 11, 8323–8338. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.S.; Pessoa, C.A.; Mello, L.D.; Kubota, L.T. Direct electron transfer: An approach for electrochemical biosensors with higher selectivity and sensitivity. J. Braz. Chem. Soc. 2003, 14, 230–243. [Google Scholar] [CrossRef]
- Rhieu, S.Y.; Reipa, V. Tuning the size of gold nanoparticles with repetitive oxidation-reduction cycles. Am. J. Nanomater. 2015, 3, 15–21. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Golestaneh, M. Simultaneous determination of sunset yellow and tartrazine in soft drinks using gold nanoparticles carbon paste electrode. Food Chem. 2012, 132, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Dorraji, P.S.; Jalali, F. Electrochemical fabrication of a novel ZnO/cysteic acid nanocomposite modified electrode and its application to simultaneous determination of sunset yellow and tartrazine. Food Chem. 2017, 227, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Cheng, Q.; Wu, K.; Yang, X. Cu-BTC frameworks-based electrochemical sensing platform for rapid and simple determination of Sunset yellow and Tartrazine. Sens. Actuators B Chem. 2016, 231, 12–17. [Google Scholar] [CrossRef]
- Deng, K.; Li, C.; Li, X.; Huang, H. Simultaneous detection of sunset yellow and tartrazine using the nanohybrid of gold nanorods decorated graphene oxide. J. Electroanal. Chem. 2016, 780, 296–302. [Google Scholar] [CrossRef]
- Arvand, M.; Gaskarmahalleh, A.A.; Hemmati, S. Enhanced-Oxidation and Highly Sensitive Detection of Tartrazine in Foodstuffs via New Platform Based on Poly (5-Sulfosalicylic Acid)/Cu(OH)2 Nanoparticles. Food Anal. Methods 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.; Shan, Y.; Xu, L.; Wu, G.; Lu, X. Development and application of the tartrazine voltammetric sensors based on molecularly imprinted polymers. Int. J. Polym. Anal. Charact. 2017, 22, 83–91. [Google Scholar] [CrossRef]







| Ratios | Percent of Interference (%) | ||||
|---|---|---|---|---|---|
| Glucose | Sucrose | Ascorbic Acid | Phenol | Sunset Yellow | |
| 1:0.01 | −0.014 | 0.393 | 0.232 | −0.043 | −0.368 |
| 1:0.1 | 0.123 | 0.363 | 0.504 | 0.045 | 0.285 |
| 1:1 | 0.772 | 1.907 | 0.808 | 0.207 | 0.615 |
| 1:10 | 0.158 | 1.616 | 0.624 | 0.194 | 0.458 |
| 1:100 | 0.340 | 1.022 | −0.592 | −0.002 | 0.325 |
| Samples | Spiked (μM) | Biosensor (μM) | HPLC (μM) | Recovery (%) |
|---|---|---|---|---|
| Candy coated with chocolate | 2 | 2.16 | 2.07 | 108.00 |
| 4 | 3.79 | 3.93 | 94.75 | |
| 6 | 6.11 | 6.03 | 101.83 | |
| Commercial mango juice | 2 | 1.91 | 1.86 | 95.50 |
| 4 | 4.09 | 4.12 | 102.25 | |
| 6 | 5.87 | 6.06 | 97.83 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazlan, S.Z.; Lee, Y.H.; Hanifah, S.A. A New Laccase Based Biosensor for Tartrazine. Sensors 2017, 17, 2859. https://doi.org/10.3390/s17122859
Mazlan SZ, Lee YH, Hanifah SA. A New Laccase Based Biosensor for Tartrazine. Sensors. 2017; 17(12):2859. https://doi.org/10.3390/s17122859
Chicago/Turabian StyleMazlan, Siti Zulaikha, Yook Heng Lee, and Sharina Abu Hanifah. 2017. "A New Laccase Based Biosensor for Tartrazine" Sensors 17, no. 12: 2859. https://doi.org/10.3390/s17122859
APA StyleMazlan, S. Z., Lee, Y. H., & Hanifah, S. A. (2017). A New Laccase Based Biosensor for Tartrazine. Sensors, 17(12), 2859. https://doi.org/10.3390/s17122859