Abstract
:1. Introduction
2. Sensor Poisoning
3. Gas Sensors Embedded in Micro-Reactor Systems
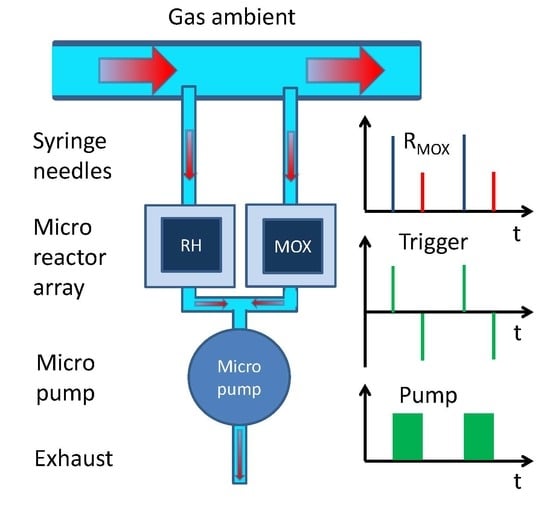
- A micro-chamber whose interior is separated from the ambient air by narrow gas inlet and outlet ports (Figure 4a,d);
- A MOX gas sensor embedded inside the micro-chamber (Figure 4b);
- A heatable catalyzer co-embedded inside the micro-chamber (Figure 4a,c);
- A micro-pump that allows the air inside the micro-chamber to be withdrawn and to be replaced by outside air that may or may not contain reactive gas components.
- In an actively forced flow-through mode (pump on);
- In an effectively separated no-flow mode (pump off).
4. Micro-Reactor-Based Gas Detection
5. Poisoning Detection
6. Possibilities and Limitations of the Micro-Reactor Approach
- (i)
- a normal observational mode, and
- (ii)
- a self-test mode.
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix B
References
- Williams, D.E. Conduction and gas response of semiconductor gas sensors. In Solid State Gas Sensors; Moseley, P.T., Tofield, P.T., Eds.; Adam Hilger: Bristol, UK, 1987; pp. 154–196. ISBN 0-85274-514-1. [Google Scholar]
- Ihokura, K.; Watson, J. The Stannic Oxide Gas Sensor—Principles and Applications; CRC Press: Boca Raton, FL, USA, 1994; ISBN 9780849326042. [Google Scholar]
- Morrison, S.R.; Sze, S.M. Semiconductor Sensors; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Comini, E.; Faglia, G.; Sberveglieri, G. Solid State Gas Sensing; Springer Science & Business Media: Boston, MA, USA, 2009; ISBN 978-0-387-09664-3. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Gas Sensor Materials: Properties, Advantages and Shortcomings for Applications; Volume 2: New Trends and Technologies; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-7387-9. [Google Scholar]
- Gardner, J.W.; Bartlett, P.N. Electronic Noses: Principles and Applications; Oxford University Press: Oxford, UK, 1999; ISBN 0-19-85595-5-0. [Google Scholar]
- Suehle, J.S.; Cavicchi, R.E.; Gaitan, M.; Semancik, S. Tin oxide gas sensor fabricated using CMOS micro-hotplates and in-situ processing. IEEE Electron Device Lett. 1993, 14, 118–120. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Hellmich, W.; Müller, G. Silicon Hotplates for Metal Oxide Gas Sensor Elements. Microsyst. Technol. 1997, 3, 183–190. [Google Scholar] [CrossRef]
- Menzel, R.; Goschnick, J. Gradient gas sensor microarrays for on-line process control—A new dynamic classification model for fast and reliable air quality assessment. Sens. Actuators B Chem. 2000, 68, 115–122. [Google Scholar] [CrossRef]
- Gardner, J.W.; Covington, J.A.; Udrea, F.; Dogaru, T.; Lu, C.C.; Milne, W. SOI-based micro-hotplate microcalorimeter gas sensor with integrated BiCMOS transducer. In Proceedings of the 11th International Conference on Solid-State Sensors and Actuators, Munich, Germany, 10–14 June 2001; pp. 1688–1691. [Google Scholar]
- Müller, G.; Friedberger, A.; Kreisl, P.; Ahlers, S.; Schulz, O.; Becker, T. A MEMS Toolkit for metal-oxide-based gas sensing systems. Thin Solid Films 2003, 436, 34–45. [Google Scholar] [CrossRef]
- Kunt, T.A.; McAvoy, T.J.; Cavicchi, R.E.; Semancik, S. Optimization of temperature programmed sensing for gas identification using micro-hotplate sensors. Sens. Actuators B Chem. 1998, 53, 24–43. [Google Scholar] [CrossRef]
- Semancik, S.; Cavicchi, R.E.; Wheeler, M.C.; Tiffany, J.E.; Poirier, G.E.; Walton, R.M.; Suehle, J.S.; Panchapakesan, B.; DeVoe, D.L. Microhotplate Platforms for Chemical Sensor Research. Sens. Actuators B Chem. 2001, 39, 579–591. [Google Scholar] [CrossRef]
- Sayhan, I.; Helwig, A.; Becker, T.; Müller, G.; Elmi, I.; Zampolli, S.; Padilla, M.; Marco, S. Discontinuously operated metal oxide gas sensors for flexible tag microlab applications. IEEE Sens. J. 2008, 8, 176–181. [Google Scholar] [CrossRef]
- Krivetski, V.; Efitorov, A.; Arkhipenko, A.; Vladimirova, S.; Rumyantseva, M.; Dolenko, S.; Gaskov, A. Selective detection of individual gases and CO/H2 mixture at low concentrations in air by single semiconductor metal oxide sensors working in dynamic temperature mode. Sens. Actuators B Chem. 2018, 254, 502–513. [Google Scholar] [CrossRef]
- Prades, J.D.; Jimenez-Diaz, R.; Hernandez-Ramirez, F.; Barth, S.; Cirera, A.; Romano-Rodriguez, A.; Mathur, S.; Morante, J.R. Ultralow power consumption gas sensors based on self-heated individual nanowires. Appl. Phys. Lett. 2008, 93, 123110. [Google Scholar] [CrossRef]
- Prades, J.D.; Jimenez-Diaz, R.; Hernandez-Ramirez, F.; Cirera, A.; Romano-Rodriguez, A.; Morante, J.R. Harnessing self-heating in nanowires for energy efficient, fully autonomous and ultra-fast gas sensors. Sens. Actuators B Chem. 2010, 144, 1–5. [Google Scholar] [CrossRef]
- Monereo, O.; Casals, O.; Prades, J.D.; Cirera, A. Self-heating in pulsed mode for signal quality improvement: Application to carbon nanostructures-based sensors. Sens. Actuators B Chem. 2016, 226, 254–265. [Google Scholar] [CrossRef]
- Monereo, O.; Illera, S.; Varea, A.; Schmidt, M.; Sauerwald, T.; Schütze, A.; Cirera, A.; Prades, J.D. Localized self-heating in large arrays of 1D nanostructures. Nanoscale 2016, 8, 5082–5088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabrega, C.; Casals, O.; Hernadez-Ramirez, F.; Prades, J.D. A review on efficient self-heating in nanowire sensors: Prospects for very low power devices. Sens. Actuators B Chem. 2018, 256, 797–811. [Google Scholar] [CrossRef]
- Chilton, J.E.; Baran, J.N.; Thomas, W.E.; Hofer, L.J.; Snyder, J.L. Silicone vapor poisoning of catalytic methane sensors. In Proceedings of the 4th WVU Conference on Coal Mine Electrotechnology, Morgantown, VA, USA, 2–4 August 1978. [Google Scholar]
- Pratt, K.F.E.; Williams, D.E. Self-Diagnostic Gas Sensitive Resistors in Sour Gas Applications. Sens. Actuators B Chem. 1997, 45, 147–153. [Google Scholar] [CrossRef]
- Helwig, A.; Müller, G.; Wassner, W.; Eickhoff, M.; Sberveglieri, G.; Fagila, G. Analysis of the Baseline Drift Phenomenon in Nano-Crystalline SnO2 Gas Sensing Layers. In Proceedings of the 11th International Meeting on Chemical Sensors, Brescia, Italy, 16–19 July 2006. [Google Scholar]
- Ahlers, S.; Müller, G.; Becker, T.; Doll, T. Factors Influencing the Gas Sensitivity of Metal Oxide Materials. In Encyclopedia of Sensors; Grimes, C.A., Dickey, E.C., Pisho, M.V., Eds.; American Scientific: Valencia, CA, USA, 2006. [Google Scholar]
- Matsubara, I.; Murayama, N.; Matsumiya, M.; Shin, W.; Qiu, F.; Izu, N. Poisoning of platinum thin film catalyst by hexamethyldisiloxane (HMDS) for thermoelectric hydrogen gas sensor. Sens. Actuators B Chem. 2003, 96, 516–522. [Google Scholar] [CrossRef]
- Rettig, F.; Moos, R.; Plog, C. Poisoning of Temperature Independent Resistive Oxygen Sensors by Sulfur Dioxide. J. Electroceramics 2004, 13, 733–738. [Google Scholar] [CrossRef]
- RAE Systems Inc. Handling LEL Sensor Poisons. Technical Note. Available online: www.raesystems.com (accessed on 16 June 2010).
- Tournier, G.; Pijolat, C. Selective filter for SnO2 based gas sensors: Application to hydrogen trace detection. Sens. Actuators B Chem. 2005, 106, 553–562. [Google Scholar] [CrossRef]
- Reimann, P.; Dausend, A.; Schütze, A. A Self-monitoring and Self-diagnosis Strategy for Semiconductor Gas Sensor Systems. IEEE Sens. 2008, 2008, 192–195. [Google Scholar] [CrossRef]
- Schüler, M.; Sauerwald, T.; Schütze, A. A novel approach for detecting HMDSO poisoning of metal oxide gas sensors and improving their stability by temperature cycled operation. J. Sens. Sens. Syst. 2015, 4, 305–311. [Google Scholar] [CrossRef]
- Fleischer, M.; Simon, E.; Rumpel, E.; Ulmer, E.; Harbeck, M.; Wandel, M.; Fietzeck, C.; Weimar, U.; Meixner, H. Detection of Volatile Compounds Correlated to Human Diseases through Breath Analysis with Chemical Sensors. Sens. Actuators B Chem. 2002, 83, 245–249. [Google Scholar] [CrossRef]
- Hackner, A.; Oberpriller, H.; Hechtenberg, V.; Müller, G. Heterogeneous Sensor Arrays: Merging Cameras and Gas Sensors into Innovative Fire Detection Systems. Sens. Actuators B Chem. 2016, 231, 497–505. [Google Scholar] [CrossRef]
- Vomiero, A.; Ponzoni, A.; Comini, E.; Ferroni, M.; Faglia, G.; Sberveglieri, G. Direct integration of metal oxide nanowires into an effective gas sensing device. Nanotechnology 2010, 21, 145502. [Google Scholar] [CrossRef] [PubMed]
- Sberveglieri, G. Recent developments in semiconducting film gas sensors. Sens. Actuators B Chem. 1995, 23, 103–109. [Google Scholar] [CrossRef]
- Krivetskiy, V.; Ponzoni, A.; Comini, E.; Badalyan, S.; Rumyantseva, M.; Gaskov, A. Selectivity Modification of SnO2-Based Materials for Gas Sensor Arrays. Electroanalysis 2010, 22, 2809–2816. [Google Scholar] [CrossRef]
- Becker, T.; Mühlberger, S.; Bosch-von Braunmühl, C.; Müller, G.; Ziemann, T.; Hechtenberg, K.V. Air pollution monitoring using tin-oxide-based microreactor systems. Sens. Actuators B Chem. 2000, 69, 108–119. [Google Scholar] [CrossRef]
- Becker, T.; Mühlberger, S.; Bosch-von Braunmühl, C.; Müller, G.; Meckes, A.; Benecke, W. Microreactors and microfluidic systems: An innovative approach to gas sensing using tin oxide-based gas sensors. Sens. Actuators B Chem. 2001, 77, 48–54. [Google Scholar] [CrossRef]
- Shaposhnik, A.; Ryabtsev, S.; Zviagin, A.; Korchagina, S.; Meshkova, N.; Shaposhnik, D.; Vasiliev, A. Selective detection of ammonia and its derivatives using MOX-sensor and microreactor. Procedia Eng. 2011, 25, 1097–1100. [Google Scholar] [CrossRef]
- Maurer, S.; Makarov, R.; Holl, G.; Kaul, P. Heterogenes Sensorsystem zum Nachweis von Explosivstoff-typischen Merkmalen durch thermische Aktivierung. In Proceedings of the Dresdner Sensor Symposium, Dresden, Germany, 7–9 December 2015; Available online: https://www.researchgate.net/publication/286453345_Heterogenes_Sensorsystem_zum_Nachweis_von_Explosivstoff-typischen_Merkmalen_durch_thermische_Aktivierung (accessed on 14 January 2018). [CrossRef]
- Konstantynovski, K.; Njio, G.; Börner, F.; Lepcha, A.; Fischer, T.; Holl, G.; Mathur, S. Bulk detection of explosives and development of customized metal oxide semiconductor gas sensors for the identification of energetic materials. Sens. Actuators B Chem. 2018, in press. [Google Scholar] [CrossRef]
- Hellmich, W.; Bosch-von Braunmühl, C.; Müller, G.; Sberveglieri, G.; Berti, M.; Perego, C. The kinetics of formation of gas-sensitive RGTO—SnO2 films. Thin Solid Films 1995, 263, 231–237. [Google Scholar] [CrossRef]
- Friedberger, A.; Kreisl, P.; Rose, E.; Müller, G.; Kühner, G.; Wöllenstein, J.; Böttner, H. Micromechanical fabrication of robust low-power metal-oxide gas sensors. Sens. Actuators B Chem. 2003, 93, 345–349. [Google Scholar] [CrossRef]
- Spannhake, J.; Helwig, A.; Schulz, O.; Müller, G. Micro-Fabrication of Gas Sensors. In Solid State Gas Sensing; Comini, E., Faglia, G., Sberveglieri, G., Eds.; Springer: Berlin, Germany, 2009; pp. 1–46. [Google Scholar] [CrossRef]
- Maier, K.; Helwig, A.; Müller, G. Towards MEMS Pellistor Spectrometers. Procedia Eng. 2015, 120, 142–145. [Google Scholar] [CrossRef]
- UFT75AT Capacitive Humidity Sensor. Data Sheet. Available online: https://www.meltec.biz/media/docs/uft75-at_bt-datenblatt.pdf (accessed on 1 February 2018).
- Maier, K.; Helwig, A.; Müller, G. Room-temperature Accumulation Gas Sensors with Periodic Reset. Sens. Actuators B Chem. 2017, 244, 701–708. [Google Scholar] [CrossRef]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helwig, A.; Hackner, A.; Müller, G.; Zappa, D.; Sberveglieri, G. Self-Test Procedures for Gas Sensors Embedded in Microreactor Systems. Sensors 2018, 18, 453. https://doi.org/10.3390/s18020453
Helwig A, Hackner A, Müller G, Zappa D, Sberveglieri G. Self-Test Procedures for Gas Sensors Embedded in Microreactor Systems. Sensors. 2018; 18(2):453. https://doi.org/10.3390/s18020453
Chicago/Turabian StyleHelwig, Andreas, Angelika Hackner, Gerhard Müller, Dario Zappa, and Giorgio Sberveglieri. 2018. "Self-Test Procedures for Gas Sensors Embedded in Microreactor Systems" Sensors 18, no. 2: 453. https://doi.org/10.3390/s18020453
APA StyleHelwig, A., Hackner, A., Müller, G., Zappa, D., & Sberveglieri, G. (2018). Self-Test Procedures for Gas Sensors Embedded in Microreactor Systems. Sensors, 18(2), 453. https://doi.org/10.3390/s18020453