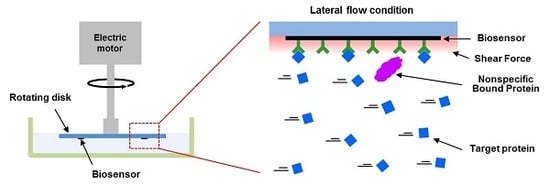
3.1. Enhanced Biosensing Speed and Improved Binding Affinity of Interacting Proteins in a High-Speed Lateral Flow System
Figure 2A shows the fluorescence image of a bare SiO
2 substrate (left) and a biotinylated SiO
2 substrate (right) after reactions with FITC-labeled streptavidin molecules under static conditions. The reactions were conducted for 90 min, and then the fluorescence images of both substrates were obtained in PBS solution by a fluorescence microscope. The biotinylated substrate exhibited much brighter fluorescence intensities than the bare SiO
2 substrate. Since fluorescence intensities are correlated with the amount of bound FITC-labeled streptavidin molecules on the biotinylated substrates, the results indicate that the biotinylated substrate could effectively capture streptavidin molecules, as we expected.
We also conducted a reaction experiment under lateral flow conditions.
Figure 2B shows the fluorescence image of bare and biotinylated SiO
2 substrates after reaction with streptavidin molecules for 90 min. In the experiment, we fixed the biotinylated substrates 15 mm away from the center of the rotating disk, and the disk was rotated at 150 rpm to achieve a lateral flow speed of ~120 mm/s in a target solution on the biotinylated substrate. Note that the biotinylated substrate under lateral flow conditions exhibited higher fluorescence intensities than that under static conditions. This indicates that the lateral flows of target solutions could increase the binding events between target molecules and their sensing molecules on a substrate.
Figure 2C shows the fluorescence intensity profiles of the bare and the biotinylated SiO
2 substrates under static (red line) and lateral (black line) flow conditions. The profiles were obtained along a black-dotted line in
Figure 2A and a red-dotted line in
Figure 2B. The average fluorescence intensities of the biotinylated substrate are about 14,100 under static conditions and about 18,570 under lateral flow conditions. These results indicate a 31.7% increase of bound streptavidin molecules on the biotinylated substrate simply as a result of flowing the target solution laterally. Interestingly, the average fluorescence intensities of the bare SiO
2 substrates are ~8440 under static conditions and ~6860 under lateral flow conditions, which indicates an 18.7% decrease of bound target molecules as a result of the lateral flow of the target solution on the bare SiO
2 substrates. These results indicate that the lateral flow of the target solution can not only increase the specific bindings on the biotinylated substrate but also decrease the nonspecific adsorptions on the bare SiO
2 surface.
Figure 2D shows the fluorescence intensity graph of biotinylated substrates after reactions with FITC-labeled streptavidin molecules under the lateral flows with the different speeds. To achieve the lateral flow speeds of 0, 30, 120 and 250 mm/s, we fixed the biotinylated substrates at 15 mm from the center of the rotating disk, and rotated the disk at 0, 40, 150 and 300 rpm. The graph shows the increase of fluorescence intensities as the lateral flow speed increases. Previously, it was reported that the binding speed of target molecules on a sensing substrate in a rotating disk system was improved as the rotating speed of the disk increased [
17,
18], which was consistent with our results. In the previous works, the rotation of the disk improved the mass transfer rate of the target molecules to the sensing substrate, thereby increasing the amount of bound target molecules on the sensing substrate. In our strategy, the sensing substrates were attached to the rotating disk at 15 mm, where high lateral flows with 120 mm/s were generated. Therefore, the effects of lateral flows on the binding kinetics between streptavidin and a biotinylated substrate also need to be considered.
To investigate only the effects of the lateral flows of a target solution on binding reactions between streptavidin and a biotinylated substrate, we performed binding reactions by changing the biotinylated substrate positions (0, 3 and 15 mm) on a rotating disk, but without changing the rotating speed of the disk.
Figure 2E shows the real-time fluorescence measurement data obtained from biotinylated SiO
2 substrates during reactions with FITC fluorescent-labeled streptavidin molecules. In this experiment, the biotinylated substrates were fixed at 0, 3 and 15 mm from the center of the disk, and the disk was rotated at 150 rpm to achieve the lateral flow of the target solution with speeds of 0, 25 and 120 mm/s, respectively. To evaluate the sensing speed, the data were fitted by the first-order rate equation:
where,
A(
t),
Amax, and τ indicate the amount of bound target molecules on a biosensor at time t, the maximum amount of bound target molecules on the biosensor, and the characteristic time constant of the reaction, respectively [
19]. By fitting the data, we were able to estimate the time constants τ of the biotinylated substrates at 0, 3 and 15 mm as τ
0 ~ 25 min, τ
3 ~ 10 min and τ
15 ~ 10 min, respectively. Note that the time constants of the substrates at 3 and 15 mm from the disk center were reduced by 60% compared with those of the substrates at the center, although the substrates at the disk center should have a larger vertical flow. It was reported previously that if there are convective flows toward the sensing substrate, the local velocity of flows is zero at the center of the substrate [
20]. Presumably, the zero velocity at the center of the rotating disk could reduce the rate of reactions due to the mass transfer rate of target molecules attenuated by the zero-flow velocity. On the other hand, the flow velocities in regions other than the center of the disk are not zero, due to the lateral flows of the target solution. Therefore, the sensing substrate at 3 and 15 mm showed higher sensing speed than the sensing substrate at 0 mm. Moreover, it should be noted that the characteristic time of a streptavidin-biotin binding reaction was calculated from the saturation time of the reactions in order to remove unexpected errors resulting from fast reaction speeds at an initial stage. Since our results show the effect of lateral flows on the sensing speed of a biosensing system, the enhanced sensing speeds can also be achieved in various biosensing systems by the lateral flow strategy.
Figure 2F shows the fluorescence intensities of biotinylated substrates at different distances from the center of a rotating disk at a reaction time of 25 min. The fluorescence intensities of the biotinylated substrates at 0, 3 and 15 mm from the disk center were 0.632 ± 0.03, 1.064 ± 0.05 and 1.246 ± 0.04, respectively. Note that the substrates with a larger lateral flow exhibited a larger binding of target molecules, even with the same reaction time, indicating enhanced binding events of target molecules as a result of lateral solution flow. We simulated the lateral and vertical flow speeds of the target solution (
Supplementary Figure S1). We found that the lateral flow speeds were proportional to the distances from the center of the disk, while the vertical flow speeds remained almost unchanged. Previously, it was reported that the amount of bound target molecules could be increased by increasing the collision frequency between target molecules and a sensing substrate [
21]. Presumably, in our works, the larger lateral flow speed of a target solution on the sensing substrate at 15 mm could increase the collision frequency of target molecules to the sensing substrate more than that at 3 mm. Therefore, higher fluorescence intensities on the sensing substrate at 15 mm were observed than on the sensing substrate at 3 mm. We also simulated the binding events between target molecules and sensing substrates located at 0, 3, 9 and 15 mm of the disk (
Supplementary Figure S2) and obtained simulation results consistent with the experimental results in
Figure 2F. The simulation results also show the turbulent flows of a target solution over 15 mm from the disk center, indicating that 15 mm from the disk center is the outermost position at which the sensing substrate can be located.
Figure 3A shows the absorbance values of bound IL-13 antigens to IL-13 antibody-coated substrate under static (black dot) and lateral (red dot) flow conditions. We loaded eight IL-13 antibody-coated substrates on the rotating disk and performed the reaction experiments in IL-13 antigen target solution with and without lateral flows (120 mm/s). During the reaction process, each reacted substrate was collected from the disk at various reaction times, and the absorbance values of the reacted substrates were obtained by using an ELISA method (see the Materials and Methods section). The absorbance data points were fitted using Equation (1). The graph shows that the responses under lateral flow conditions exhibited higher absorbance values than those under static conditions. Since absorbance values indicate the amount of bound IL-13 antigens to the antibody-coated substrates, the results show that the amount of bound IL-13 antigens increased as a result of the lateral flows of the target solution even in an immune protein reaction. Based on these results, we could expect that the lateral flow strategy could be applied in various sensing applications simply by changing the sensing substrates on a rotating disk.
Figure 3B shows the dose-response of IL-13 antibody-coated substrates to IL-13 antigens under static and lateral flow conditions. The reaction experiments were conducted in IL-13 antigen solution for 2 h with the concentration range of the IL-13 antigen solution from 0.01 to 100 ng/mL. The experimental data points were fitted using Hill’s equation [
22]. Under static conditions, the equilibrium dissociation constant K of IL-13 was about 1.18 ± 0.1 ng/mL, which was consistent with the previously reported value [
23]. However, under lateral flow conditions, the constant K of IL-13 was about 0.34 ± 0.08 ng/mL, which was three times lower than that observed under static conditions. This indicates an enhanced binding affinity in an immune protein reaction as a result of the lateral flow of the target solution, which has not been reported before. We also estimated the limit of detection (LOD) from
Figure 3B. The limit of detection is defined as the concentration yielding a signal equal to the blank signal plus three times its standard deviation [
17,
18]. Based on the results of IL-13 tests, LODs of 69 ± 1 pg/mL and 21 ± 0.3 pg/mL under static and lateral flow conditions, respectively, were obtained. These results clearly show the enhanced LOD under the lateral flow condition.
3.2. Reduced Nonspecific Adsorptions
Figure 4A shows the fluorescence images of bare and biotinylated SiO
2 substrates (i) before reactions with FITC-labeled streptavidin molecules, (ii) after reaction under static conditions for 90 min, and (iii) after reaction under static and lateral flow conditions for 170 min. The reaction experiments were sequentially performed under a static condition for 90 min, and then under a lateral flow condition for 80 min. Before reactions with FITC-labeled streptavidin molecules (
Figure 4(Ai)), only background fluorescence intensities were measured on both the bare and the biotinylated substrates. After reaction under static conditions (
Figure 4(Aii)), clear differences in fluorescence intensities between bare and biotinylated substrates were observed. Interestingly, after reaction under lateral flow conditions (
Figure 4(Aiii)), the fluorescence intensities of the biotinylated substrate increased, while the fluorescence intensities of the bare SiO
2 substrate slightly decreased compared to those of the bare SiO
2 substrate in
Figure 4(Aii). The results show that the lateral flow increases the specific binding reactions between streptavidin and biotin molecules, while decreasing the nonspecific bindings of streptavidin molecules. These results are consistent with the results of
Figure 2C, indicating the reliability of our observations.
Figure 4B shows the real-time response curves of bare (black dot) and biotinylated (red dot) substrates during reaction with FITC-labeled streptavidin molecules. Here, the same experimental conditions as the experiments depicted in
Figure 4A were used for the real-time response experiments. The experimental data of biotinylated substrates under static conditions were fitted using Equation (1), and the fitted curve is marked in a blue line. Black dots in the figure represent the fluorescence intensities of bare SiO
2 substrates, which indicate the amount of the nonspecific adsorptions of streptavidin molecules. The streptavidin molecules specifically bound to the biotinylated substrates were almost saturated for 90 min under static conditions and increased again when we applied the lateral flows on the substrates. Note that the fluorescence intensities of bare SiO
2 substrates showed negligible changes as a result of the lateral flow. This result indicates that the enhanced binding reactions such as those between streptavidin and biotin molecules under lateral flow conditions did not increase the nonspecific adsorptions of streptavidin molecules.
Figure 4C shows the nonspecific binding reaction curves of FITC-labeled streptavidin molecules to bare SiO
2 substrates under static and lateral flow conditions. To obtain only nonspecifically bound streptavidin molecules, bare SiO
2 substrates were reacted with FITC-labeled streptavidin molecules with and without lateral flows (120 mm/s) for 90 min. Black and red dots in the graph indicate experimental data under static and lateral flow conditions, respectively. The graph shows the fluorescence intensities of bare SiO
2 substrates reacted with FITC-labeled streptavidin molecules under static conditions increase as reaction time increase. On the other hand, under lateral flow conditions, the fluorescence intensities of a bare SiO
2 substrate reacted with FITC-labeled streptavidin molecules rapidly increase over 40 min. Then the fluorescence intensities are saturated. The saturated intensities are lower than the fluorescence intensities under static conditions. This implies that the lateral flows of a target solution hinder the nonspecific adsorptions of streptavidin molecules.
Figure 4D shows the dissociation curves of nonspecifically adsorbed FITC-labeled streptavidin molecules on bare SiO
2 substrates under static and lateral flow conditions. For the nonspecific adsorptions of streptavidin molecules on bare SiO
2 substrates, bare SiO
2 substrates were incubated in 1 µg/mL streptavidin solution for 12 h at 4 °C. The incubated substrates were gently washed with a PBS solution, and the tests were conducted in PBS solution under static and lateral flow conditions for 60 min. The measured fluorescence intensities were fitted using exponential decline functions [
24]. Black and red lines indicate the fitting curves of the static and lateral flow condition experiments, respectively. The graph shows that fluorescence intensities decreased significantly under lateral flow conditions, while they barely changed under static conditions. These results clearly indicate that nonspecifically adsorbed streptavidin molecules can be reduced by lateral flows. Considering that the nonspecific binding of molecules reduces the specificity of a biosensing system, the lateral flow strategy could be used to enhance the specificity of a biosensing system.
A plausible explanation for the reduced non-specific reactions could be shear forces under lateral flow conditions. To estimate the shear forces generated by lateral flows in our experiments, we first conducted the simulation of shear rate distributions around a rotating disk with a 10 nm-sized particle 15 mm from the disk’s center (
Supplementary Figure S3). The shear force near the particle was estimated by multiplying the shear rate with the viscosity of water. The estimated shear force on a 10 nm-sized particle was ~2.7 pN. This corresponds to only ~1% of the reported binding force (220~460 pN) of a single streptavidin-biotin pair [
25,
26,
27]. On the other hand, it is ~10% of the common nonspecific binding forces (20~100 pN) between streptavidin molecules and a glass substrate [
27]. Since a streptavidin molecule has four biotin binding sites, the specific binding force of a single streptavidin to biotin molecules would be higher than what has been reported. Therefore, the shear forces generated by a lateral flow might significantly affect only nonspecific bindings of streptavidin molecules. These results are summarized in
Table 1. Since the nonspecific bindings of target molecules could result in background noise or false signals in biosensors, our strategy could be used to enhance the selectivity of bio-sensing systems.
3.3. Improved Binding Affinity under Different pH and Ionic Strength Conditions
Figure 5A shows the normalized fluorescence intensities of bound FITC-labeled streptavidin molecules on biotinylated substrates under different pH and flow speed conditions. In order to prepare streptavidin solutions with various pH values, we dissolved FITC-labeled streptavidin powders in citric acid-Na
2HPO
4 buffer solutions that had been titrated to have pH ranges from 3 to 7. The sensing experiments were conducted under static and lateral flow conditions in different pH value target solutions for 2 h. The obtained fluorescence intensities were normalized with respect to the result at pH 7. The fluorescence intensities of bound FITC-labeled streptavidin molecules under static conditions (black bars) decrease with the decrease in pH value. Previous work has shown that under low pH conditions, many sensing molecules lose their binding affinity, and sensor devices based on the sensing molecules do not work [
28,
29,
30], which is consistent with our results. On the other hand, it should be pointed out that the fluorescence intensities under lateral flow conditions (red bars) were almost the same over the entire pH range, indicating the same amount of bound streptavidin on the biotinylated substrate. These results show that under lateral flow conditions, biotins on the substrate maintain their affinity to streptavidin, and that the biotinylated substrate can be used as a sensor device even in target solutions with very low pH.
We also performed a similar experiment with FITC-labeled streptavidin binding onto biotinylated substrates under different ionic strength and flow speed conditions (
Figure 5B). In order to prepare streptavidin solutions with different ionic strengths, 1 M NaCl solutions were added to sodium acetate buffer solutions (pH 5) such that they had ionic strengths ranging from 3 mM to 200 mM. Then, FITC-labeled streptavidin powders were dissolved in the solutions to result in streptavidin concentrations of 1 ng/mL. The fluorescence intensities of bound FITC-labeled streptavidin molecules under static conditions (black bars) decreased with a decrease in ionic strength. Presumably, at a low ionic strength, the binding affinity of biotin onto streptavidin became weaker, as reported previously [
31]. Interestingly, fluorescence intensities under lateral flow conditions (red bars) are maintained regardless of ionic strength changes. These results imply that the binding affinity of streptavidin-biotin reactions is maintained by the lateral flows of the target solution even in low ionic strength conditions. Such an improved binding affinity under lateral flow conditions has not been reported before.
Figure 5C shows schematic diagrams depicting a plausible explanation of the improved binding affinity observed in our experiments under lateral flow conditions. It shows the movement of streptavidin molecules to a biotinylated biosensor under (i) static and (ii) lateral flow conditions in low pH or low ionic strength solutions. Previous work has shown that the decrease in binding affinity between interacting molecules in low pH or low ionic strength solutions can be explained by the net electrical charge of molecules [
32,
33]. When two interacting molecules with the same electrical charge approach each other, long-range electrostatic repulsive forces between the molecules increase, which obstruct binding interactions between the two molecules. The net electrical charges of streptavidin molecules and biotinylated SiO
2 substrates were reported to have positive values below a pH of 6 [
32,
34,
35]. These positive charges increase as pH or ionic strength decreases, enhancing the repulsive forces between the streptavidin molecules and biotinylated surfaces. Therefore, binding reactions between streptavidin and biotin molecules can be attenuated by lowering the pH or ionic strengths of a liquid medium (
Figure 5(Ci)).
On the other hand, under lateral flow conditions, as shown in
Figure 5(Cii), the lateral flows increase the molecular speed of streptavidin molecules as well as the mass transfer rates of the molecules to the biotinylated surface. Presumably, the increased molecular speeds and mass transfer rates could help in overcoming the electrostatic repulsive forces between the streptavidin molecules and the biotinylated substrate and enhance the probability of the presence of streptavidin molecules near the biotinylated surface. This could induce an increase in effective collisions between streptavidin and biotin molecules under lateral flow conditions, allowing the binding rate of streptavidin-biotin molecules to be maintained even under low pH and ionic conditions. We also performed experiments investigating the effects of a lateral flow on the binding reactions between IL-13 antigens and IL-13 antibody-coated substrates at various pH conditions and found that the binding reactions of immune proteins could also be enhanced under low pH conditions by lateral flow in the target solution (
Supplementary Figure S4). In many biosensing systems, the changes of the environmental conditions of target solutions hinder the quantitative detections of target molecules. Thus, our lateral flow strategy could also be a powerful tool for developing a quantitative biosensing system without worries about environmental conditions.