Molecular Assembly of a Durable HRP-AuNPs/PEDOT:BSA/Pt Biosensor with Detailed Characterizations
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Instruments and Electrochemical Measurements
2.3. Characterizations of Electrode Surfaces
3. Results and Discussion
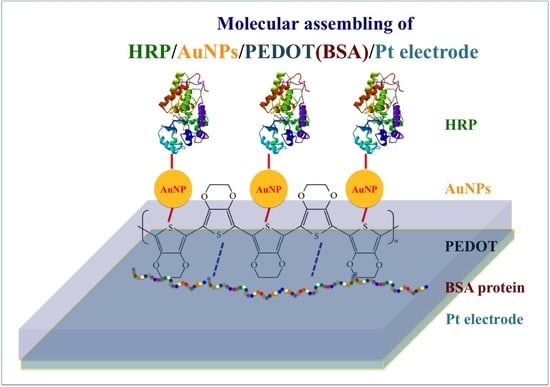
3.1. Constructing HRP-AuNPs/PEDOT:BSA/Pt Electrode
3.2. Electrochemical Measurements of H2O2
3.3. FTIR Characterization of HRP-AuNPs/PEDOT:BSA/Pt Electrode
3.4. Raman Characterization of HRP-AuNPs/PEDOT:BSA/Pt Electrode
3.5. XPS Characterization of HRP-AuNPs/PEDOT:BSA/Pt Electrode
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirsch, J.; Siltanen, C.; Zhou, Q.; Revzin, A.; Simonian, A. Biosensor technology: Recent advances in threat agent detection and medicine. Chem. Soc. Rev. 2013, 42, 8733–8768. [Google Scholar] [CrossRef] [PubMed]
- Luong, J.H.T.; Male, K.B.; Glennon, J.D. Biosensor technology: Technology push versus market pull. Biotechnol. Adv. 2008, 26, 492–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.C.; Gu, Y.S. Enhancing the sensitivity and stability of HRP/PANI/Pt electrode by implanted bovine serum albumin. Biosens. Bioelectron. 2008, 23, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Sumathi, C.; Dharuman, V.; Wilson, J. Gold nanoparticles functionalized poly(3,4-ethylenedioxythiophene) thin film for highly sensitive label free DNA detection. Anal. Methods 2013, 5, 684–689. [Google Scholar] [CrossRef]
- Gu, Y.; Lai, M.T. The potential application of a poly(3,4-ethylenedioxythiopene) modified platinum DNA biosensor in mutation analysis. Biosens. Bioelectron. 2012, 31, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.C.; Xie, H.; Chen, N.Y.; Yu, H.H. Trinity DNA Detection Platform by Ultrasmooth and Functionalized PEDOT Biointerfaces. ACS Appl. Mater. Interfaces 2009, 1, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.C.; Ren, S.B.; Gu, Y.S. A Novel Conductive Poly(3,4-ethylenedioxythiophene)-BSA Film for the Construction of a Durable HRP Biosensor Modified with NanoAu Particles. Sensors 2016, 16, 374. [Google Scholar] [CrossRef] [PubMed]
- Akita, M.; Tsutsumi, D.; Kobayashi, M.; Kise, H. Structural change and catalytic activity of horseradish peroxidase in oxidative polymerization of phenol. Biosci. Biotechnol. Biochem. 2001, 65, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Do, J.S.; Gu, Y. Immobilization of HRP in Mesoporous Silica and Its Application for the Construction of Polyaniline Modified Hydrogen Peroxide Biosensor. Sensors 2009, 9, 4635–4648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.S.; Chen, C.C.; Ruan, Z.W. Enzymatic synthesis of conductive polyaniline using linear BSA as the template in the presence of sodium dodecyl sulfate. Synth. Met. 2009, 159, 2091–2096. [Google Scholar] [CrossRef]
- Wang, J.; Gu, Y. Extraction and characterizations of enzymatically synthesized conductive poly(3,4-ethylenedioxythiophene). J. Taiwan Inst. Chem. Eng. 2014, 45, 1170–1175. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Ramanaviciene, A. Hemoproteins in Design of Biofuel Cells. Fuel Cells 2009, 9, 25–36. [Google Scholar] [CrossRef]
- Liu, X.J.; Luo, L.Q.; Ding, Y.P.; Xu, Y.H.; Li, F. Hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase on gamma-Al2O3 nanoparticles/chitosan film-modified electrode. J. Solid State Electrochem. 2011, 15, 447–453. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Ansari, A.A.; Sumana, G.; Malhotra, B.D. Horse radish peroxidase immobilized polyaniline for hydrogen peroxide sensor. Polym. Adv. Technol. 2011, 22, 903–908. [Google Scholar] [CrossRef]
- Ansari, A.A.; Solanki, P.R.; Malhotra, B.D. Hydrogen peroxide sensor based on horseradish peroxidase immobilized nanostructured cerium oxide film. J. Biotechnol. 2009, 142, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.S.; Chen, X.; Zhang, X.; Yang, W.S.; Evans, D.G. Intercalation of methylene blue into layered manganese oxide and application of the resulting material in a reagentless hydrogen peroxide biosensor. Sens. Actuators B Chem. 2008, 129, 784–789. [Google Scholar] [CrossRef]
- Wang, T.D.; Triadafilopoulos, G.; Crawford, J.M.; Dixon, L.R.; Bhandari, T.; Sahbaie, P.; Friediand, S.; Soetikno, R.; Contag, C.H. Detection of endogenous biomolecules in Barrett’s esophagus by Fourier transform infrared spectroscopy. Proc. Natl. Acad. Sci. USA 2007, 104, 15864–15869. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.M.; Bourassa, M.W.; Smith, R.J. FTIR spectroscopic imaging of protein aggregation in living cells. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Selvaganesh, S.V.; Mathiyarasu, J.; Phani, K.L.N.; Yegnaraman, V. Chemical synthesis of PEDOT-Au nanocomposite. Nanoscale Res. Lett. 2007, 2, 546–549. [Google Scholar] [CrossRef]
- Yu, S.J.; Luo, C.H.; Wang, L.W.; Peng, H.; Zhu, Z.Q. Poly(3,4-ethylenedioxythiophene)-modified Ni/silicon microchannel plate electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Analyst 2013, 138, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Farukh, M.; Singh, A.P.; Dhawan, S.K. Enhanced electromagnetic shielding behavior of multi-walled carbon nanotube entrenched poly(3,4-ethylenedioxythiophene) nanocomposites. Compos. Sci. Technol. 2015, 114, 94–102. [Google Scholar] [CrossRef]
- Zhou, C.F.; Liu, Z.W.; Du, X.S.; Ringer, S.P. Electrodeposited PEDOT films on ITO with a flower-like hierarchical structure. Synth. Met. 2010, 160, 1636–1641. [Google Scholar] [CrossRef]
- Pakiari, A.H.; Jamshidi, Z. Nature and Strength of M-S Bonds (M = Au, Ag, and Cu) in Binary Alloy Gold Clusters. J. Phys. Chem. A 2010, 114, 9212–9221. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Ren, S.; Li, J.; Bi, X.; Gu, Y. Molecular Assembly of a Durable HRP-AuNPs/PEDOT:BSA/Pt Biosensor with Detailed Characterizations. Sensors 2018, 18, 1823. https://doi.org/10.3390/s18061823
Xu F, Ren S, Li J, Bi X, Gu Y. Molecular Assembly of a Durable HRP-AuNPs/PEDOT:BSA/Pt Biosensor with Detailed Characterizations. Sensors. 2018; 18(6):1823. https://doi.org/10.3390/s18061823
Chicago/Turabian StyleXu, Fangcheng, Shuaibin Ren, Jiansin Li, Xiang Bi, and Yesong Gu. 2018. "Molecular Assembly of a Durable HRP-AuNPs/PEDOT:BSA/Pt Biosensor with Detailed Characterizations" Sensors 18, no. 6: 1823. https://doi.org/10.3390/s18061823
APA StyleXu, F., Ren, S., Li, J., Bi, X., & Gu, Y. (2018). Molecular Assembly of a Durable HRP-AuNPs/PEDOT:BSA/Pt Biosensor with Detailed Characterizations. Sensors, 18(6), 1823. https://doi.org/10.3390/s18061823