Measurement Uncertainty Calculations for pH Value Obtained by an Ion-Selective Electrode
Abstract
:1. Introduction
2. Potentiometric Principles
3. The Principles of Uncertainty Calculations
- The analytical method. It requires very good skills in mathematical transformations of PDF and is therefore very time-consuming.
- The first-order Taylor approximation method. It is based on replacing the model by a first-order Taylor series. It is the typical method for uncertainty evaluating and is very often applied.
- The nth order Taylor approximation method. It is based on replacing the model by a higher-order Taylor series. The method is more exact, especially for nonlinear models, but requires more advanced mathematics. The possibility of making mistakes during the mathematical transformations is high.
- The numerical method. It implements the propagation of PDF, is very effective, but requires better programming skills.
4. Materials and Methods
5. Results and Discussion
5.1. Calculation of Unknown pH Value
5.2. Uncertainty Evaluation
5.2.1. Method with the Taylor Approximation
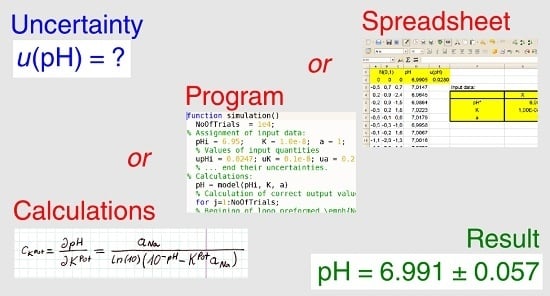
5.2.2. Numerical Method Implemented in a Program
5.2.3. Numerical Method Implemented in a Spreadsheet
5.2.4. Uncertainty Evaluation Using Dedicated Software
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GUM | Guide to the expression of Uncertainty in Measurement |
| ISE | Ion-Selective Electrode |
| MC | Monte Carlo |
| Probability Density Function | |
| VIM | International Vocabulary of Metrology |
| UI | User Interface |
Appendix A. C++ Code


Appendix B. Matlab/GNU Octave Code

Appendix C. R Code

Appendix D. Python Code

References
- Stebel, K.; Choinski, D. Performance Improvement for Quasi Periodical Disturbances in pH Control. Adv. Electr. Comput. Eng. 2015, 15, 123–134. [Google Scholar] [CrossRef]
- JCGM. Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement. 2008. Available online: https://www.bipm.org/utils/common/documents/jcgm/JCGM_100_2008_E.pdf (accessed on 12 June 2018).
- EURACHEM/CITAC. QUAM:2012.P1 Guide CG 4. Quantifying Uncertainty in Analytical Measurement, 3rd ed. 2012. Available online: https://www.eurachem.org/images/stories/Guides/pdf/QUAM2012_P1.pdf (accessed on 12 June 2018).
- Amman, D. Ion-Selective Microelectrodes; Springer: Berlin/Heidelberg, Germany, 1986; ISBN 0387162224. [Google Scholar]
- Morf, W.E. The Principles of Ion-Selective Electrodes and of Membrane Transport; Akadémiai Kiadó: Budapest, Hungary, 1981; ISBN 963-05-2511-9. [Google Scholar]
- Buck, R.P.; Rondinini, S.; Covington, A.K.; Baucke, F.G.K.; Brett, C.M.A.; Camoes, M.F.; Milton, M.J.T.; Mussini, T.; Naumann, R.; Pratt, K.W.; et al. Measurement of pH. Definition, standards, and procedures (IUPAC Recommendations 2002). Pure Appl. Chem. 2002, 74, 2169–2200. [Google Scholar] [CrossRef] [Green Version]
- Wiora, J. Problems and risks occurred during uncertainty evaluation of a quantity calculated from correlated parameters: A case study of pH measurement. Accredit. Qual. Assur. 2016, 21, 33–39. [Google Scholar] [CrossRef]
- Meinrath, G.; Spitzer, P. Uncertainties in Determination of pH. Microchim. Acta 2000, 135, 155–168. [Google Scholar] [CrossRef]
- Buchczik, D.; Ilewicz, W. Evaluation of calibration results using the least median of squares method in the case of linear multivariate models. In Proceedings of the 2016 21st International Conference on Methods and Models in Automation and Robotics (MMAR), Miedzyzdroje, Poland, 29 August–1 September 2016; pp. 800–805. [Google Scholar]
- JCGM. International Vocabulary of Metrology—Basic and General Concepts and Associated Terms (VIM), 3rd ed. 2012. Available online: https://www.bipm.org/utils/common/documents/jcgm/JCGM_200_2012.pdf (accessed on 12 June 2018).
- European Accreditation. EA-4/02 Expression of the Uncertainty of Measurement in Calibration; European Accreditation: Paris, France, 2013. [Google Scholar]
- Rubinson, K.A.; Rubinson, J.F. Contemporary Instrumental Analysis; Prentice Hall: Upper Saddle River, NJ, USA, 2000; ISBN 0137907265. [Google Scholar]
- Midgley, D.; Torrance, K. Potentiometric Water Analysis, 2nd ed.; John Wiley & Sons, Inc.: Chichester, UK, 1991; ISBN 0471929832. [Google Scholar]
- Eckschlager, K. Errors, Measurement and Results in Chemical Analysis; Van Nostrand Reinhold Co.: New York, NY, USA, 1969; ISBN 0442022301. [Google Scholar]
- Wiora, J. Improvement of measurement results based on scattered data in cases where averaging is ineffective. Sens. Actuator B-Chem. 2014, 201, 475–481. [Google Scholar] [CrossRef]
- Sharaf, M.A.; Illan, D.L.; Kowalski, B.R. Chemometrics; Chemical Analysis; John Wiley & Sons: New York, NY, USA, 1986; Volume 82, ISBN 0471831069. [Google Scholar]
- Fischer, H. A History of the Central Limit Theorem; Springer Science + Business Media: Berlin, Germany, 2011. [Google Scholar]
- Wiora, J.; Kozyra, A.; Wiora, A. A weighted method for reducing measurement uncertainty below that which results from maximum permissible error. Meas. Sci. Technol. 2016, 27, 035007. [Google Scholar] [CrossRef]
- Olsen, R.J.; Sattar, S. Measuring the Gas Constant R: Propagation of Uncertainty and Statistics. J. Chem. Educ. 2013, 90, 790–792. [Google Scholar] [CrossRef]
- Kušnerová, M.; Valíček, J.; Harničárová, M.; Hryniewicz, T.; Rokosz, K.; Palková, Z.; Václavík, V.; Řepka, M.; Bendová, M. A Proposal for Simplifying the Method of Evaluation of Uncertainties in Measurement Results. Meas. Sci. Rev. 2013, 13, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wiora, J. Uncertainty Evaluation of pH Measured Using Potentiometric Method. In Intelligent Systems’2014; Filev, D., Jabłkowski, J., Kacprzyk, J., Krawczak, M., Popchev, I., Rutkowski, L., Sgurev, V., Sotirova, E., Szynkarczyk, P., Zadrozny, S., Eds.; Advances in Intelligent Systems and Computing; Springer: Cham, Switzerland, 2015; Volume 323, pp. 523–531. [Google Scholar]
- JCGM. Evaluation of Measurement Data—Supplement 1 to the “Guide to the Expression of Uncertainty in Measurement”—Propagation of Distributions Using a Monte Carlo Method. 2008. Available online: https://www.bipm.org/utils/common/documents/jcgm/JCGM_101_2008_E.pdf (accessed on 12 June 2018).
- American Association for Laboratory Accreditation. G104—Guide for Estimation of Measurement Uncertainty In Testing; American Association for Laboratory Accreditation: Frederick, MD, USA, 2014. [Google Scholar]
- Douglas, J.E. Data analysis in the computer age: A Monte Carlo computer simulation of error sensitivity in the determination of formation constants. J. Chem. Educ. 1992, 69, 885. [Google Scholar] [CrossRef]
- Theodorou, D.; Meligotsidou, L.; Karavoltsos, S.; Burnetas, A.; Dassenakis, M.; Scoullos, M. Comparison of ISO-GUM and Monte Carlo methods for the evaluation of measurement uncertainty: Application to direct cadmium measurement in water by GFAAS. Talanta 2011, 83, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.M.; Cox, M.G. On a Monte Carlo method for measurement uncertainty evaluation and its implementation. Metrologia 2014, 51, S176. [Google Scholar] [CrossRef]
- Horne, K.; Fleming, A.; Timmins, B.; Ban, H. Monte Carlo uncertainty analysis for photothermal radiometry measurements using a curve fit process. Metrologia 2015, 52, 783. [Google Scholar] [CrossRef]
- Cho, C.; Lee, J.G.; Kim, J.H.; Kim, D.C. Uncertainty Analysis in EVM Measurement Using a Monte Carlo Simulation. IEEE Trans. Instrum. Meas. 2015, 64, 1413–1418. [Google Scholar] [CrossRef]
- Sobol, I.M. A Primer for the Monte Carlo Method, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1994; ISBN 084938673X. [Google Scholar]
- Gedam, S.G.; Beaudet, S.T. Monte Carlo simulation using Excel(R) spreadsheet for predicting reliability of a complex system. In Proceedings of the Reliability and Maintainability Symposium, Los Angeles, CA, USA, 24–27 January 2000; pp. 188–193. [Google Scholar]
- Decker, J.E.; Eves, B.J.; Pekelsky, J.R.; Douglas, R.J. Evaluation of uncertainty in grating pitch measurement by optical diffraction using Monte Carlo methods. Meas. Sci. Technol. 2011, 22, 027001. [Google Scholar] [CrossRef]
- Yanai, R.D.; Battles, J.J.; Richardson, A.D.; Blodgett, C.A.; Wood, D.M.; Rastetter, E.D. Estimating Uncertainty in Ecosystem Budget Calculations. Ecosystems 2010, 13, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Wiora, J. The Application of a Spreadsheet in the Uncertainty Evaluation Using Monte Carlo Method in the Case of Ion-Selective Electrodes; VIII Polska Konferencja Chemii Analitycznej. 2010, p. 211. (In Polish). Available online: https://http://www2.chemia.uj.edu.pl/pkca2010/doc/program.pdf (accessed on 12 June 2018).
- Chew, G.; Walczyk, T. A Monte Carlo approach for estimating measurement uncertainty using standard spreadsheet software. Anal. Bioanal. Chem. 2012, 402, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Zangl, H.; Hoermaier, K. Educational aspects of uncertainty calculation with software tools. Measurement 2017, 101, 257–264. [Google Scholar] [CrossRef]
- Microsoft Excel Function Translations. Available online: http://www.computerhope.com/issues/ch000704.htm (accessed on 23 May 2018).
- List of Uncertainty Propagation Software. Available online: http://en.wikipedia.org/wiki/List_of_uncertainty_propagation_software (accessed on 23 May 2018).
- Metrodata GmbH. Available online: http://www.metrodata.de (accessed on 23 May 2018).
- Qualisyst Ltd. Available online: http://www.qsyst.com (accessed on 23 May 2018).






| No | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| i | mV | mV | ( mV) | mV | mV | ( mV) | mV | mV | ( mV) |
| 1 | 182.4 | 0 | 0 | −103.8 | 0 | 0 | 9.0 | −3 | 9 |
| 2 | 182.6 | 2 | 4 | −103.9 | 1 | 1 | 9.2 | −1 | 1 |
| 3 | 182.2 | −2 | 4 | −104.0 | 2 | 4 | 9.3 | 0 | 0 |
| 4 | 182.1 | −3 | 9 | −103.7 | −1 | 1 | 9.6 | 3 | 9 |
| 5 | 182.7 | 3 | 9 | −103.6 | −2 | 4 | 9.4 | 1 | 1 |
| mean | 182.4 | −103.8 | 9.3 | ||||||
| SSD | 26 | 10 | 20 |
| Name | Quantity | Estimate | Standard Uncertainty | Sensitivity Coefficient | Uncertainty Contribution | Squares of Uncert. Contr. |
|---|---|---|---|---|---|---|
| Symbol | ||||||
| Column No. | (1) | (2) | (3) | (4) | (5) = (3)·(4) | (6) = |
| Sources | ||||||
| Result |
| Language | Time (s) |
|---|---|
| C++ | 6.16 |
| Octave | 1.68 |
| R | 4.72 |
| Python | 3.90 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiora, J.; Wiora, A. Measurement Uncertainty Calculations for pH Value Obtained by an Ion-Selective Electrode. Sensors 2018, 18, 1915. https://doi.org/10.3390/s18061915
Wiora J, Wiora A. Measurement Uncertainty Calculations for pH Value Obtained by an Ion-Selective Electrode. Sensors. 2018; 18(6):1915. https://doi.org/10.3390/s18061915
Chicago/Turabian StyleWiora, Józef, and Alicja Wiora. 2018. "Measurement Uncertainty Calculations for pH Value Obtained by an Ion-Selective Electrode" Sensors 18, no. 6: 1915. https://doi.org/10.3390/s18061915
APA StyleWiora, J., & Wiora, A. (2018). Measurement Uncertainty Calculations for pH Value Obtained by an Ion-Selective Electrode. Sensors, 18(6), 1915. https://doi.org/10.3390/s18061915