A Contactless Sensor for Pacemaker Pulse Detection: Design Hints and Performance Assessment
Abstract
:1. Introduction
2. Materials and Methods
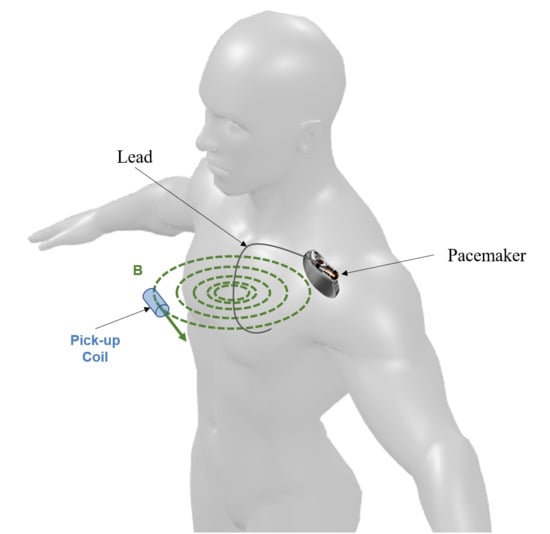
2.1. Physical Model
- The angle (γ) between the coil axis and the plane of the magnetic field line;
- The angle () between the B vector and the coil axis projection onto the plane of the magnetic field line.
2.2. Experimental Measurements of Coil Response
2.3. Assembled Sensor Architecture
2.3.1. Analog Front-End
2.3.2. Digital Unit
2.4. Assembled Sensor Performances
3. Results
3.1. Experimental Measurements of Coil Response
3.2. Assembled Sensor Performances
4. Discussion
4.1. Physical Model Validation
4.2. Assembled Sensor Performances
5. Conclusions
- the reduction of sensor dimensions and power consumption, in order to actually make it a wearable device with adequate battery life;
- the implementation of a pattern recognition approach to address the issue of the measurements corruption due to electromagnetic interferences, which is fundamental for obtaining reliable information from remote monitoring.
- the integration of the coil sensor with a heart contraction detector, in order to obtain information about the mechanical activity of the patient’s heart, allowing the identification of other important common malfunctions of pacemakers such as failure-to-capture, failure-to-pace (oversensing) and failure-to-sense (undersensing), thus improving the assessment of their correct operation. This can be achieved, for example, with the integration of wearable Bluetooth sensors [28] which would send the gathered information to the proposed pacemaker activity sensor, or to a third data acquisition system (e.g., smartphone, tablet, or another personal portable device);
- the development of an alarm device to help the patient stay away from electromagnetic interferences, which can affect the pacemaker correct operation, alerting him at a safe distance from interference sources, e.g., by providing an acoustic alarm. This is important since a majority of implanted patients are not aware of all possible interference sources, even after proper education provided by health professionals.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
References
- Mond, H.G.; Proclemer, A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: Calendar year 2009—A World Society of Arrhythmia’s project. Pacing Clin. Electrophysiol. 2011, 34, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Greenspon, A.J.; Patel, J.D.; Lau, E.; Ochoa, J.A.; Frisch, D.R.; Ho, R.T.; Pavri, B.B.; Kurtz, S.M. Trends in Permanent Pacemaker Implantation in the United States from 1993 to 2009. J. Am. Coll. Cardiol. 2012, 60, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Gregoratos, G.; Cheitlin, M.D.; Conill, A.; Epstein, A.E.; Fellows, C.; Freedman, R.A.; Hlatky, M.A.; Naccarelli, G.V.; Saksena, S.; Schlant, R.C.; et al. ACC/AHA Guidelines for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices: Executive Summary—A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Pacemaker Implantation). Circulation 1998, 97, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.R. Follow up and optimisation of cardiac pacing. Hear 2005, 91, 1229–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udo, E.O.; van Hemel, N.M.; Zuithoff, N.P.; Barrett, M.J.; Ruiter, J.H.; Doevendans, P.A.; Moons, K.G. Incidence and predictors of pacemaker reprogramming: Potential consequences for remote follow-up. Europace 2013, 15, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Schuchert, A.; Lepage, S.; Ostrander, J.J.; Bos, R.J.; Gwechenberger, M.; Nicholls, A.; Ringwald, G. Automatic analysis of pacemaker diagnostic data in the identification of atrial tachyarrhythmias in patients with no prior history of them. Europace 2005, 7, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sack, S.; Mouton, E.; Defaye, P.; Dagres, N.; Wolfhard, U.; Wieneke, H.; Herrmann, J.; Oldenburg, O.; Golles, A.; Erbel, R. AIDA Multicenter Study Group. Automatic Interpretation for Diagnosis Asistance. Improved detection and analysis of sensed and paced events in dual chamber pacemakers with extended memory function. A prospective multicenter trial in 626 patients. Herz 2001, 26, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Defaye, P.; Dournaux, F.; Mouton, E. Prevalence of supraventricular arrhythmias from the automated analysis of data stored in the DDD pacemakers of 617 patients: The AIDA study. The AIDA Multicenter Study Group. Automatic Interpretation for Diagnosis Assistance. Pacing Clin. Electrophysiol. 1998, 21, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, S.M.V.; Craveiro, N.M.; Darrieux, F.; de Oliveira, Í.M.; Scanavacca, M.; Hachul, D. Accuracy of the pacemaker event recorder versus Holter-ECG to detect both symptomatic and asymptomatic ventricular arrhythmias. J. Cardiovasc. Electrophysiol. 2018, 29, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.A.; Cleland, J.; Deharo, J.C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013, 15, 1070–1118. [Google Scholar] [PubMed] [Green Version]
- Vardas, P.E.; Auricchio, A.; Blanc, J.J.; Daubert, J.C.; Drexler, H.; Ector, H.; Gasparini, M.; Linde, C.; Morgado, F.B.; Oto, A.; et al. Guidelines for cardiac pacing and cardiac resynchronization therapy: The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in Collaboration with the European Heart Rhythm Association. Eur. Heart J. 2007, 28, 2256–2295. [Google Scholar] [PubMed] [Green Version]
- Ricke, A.D.; Swiryn, S.; Bauernfeind, R.A.; Conner, J.A.; Young, B.; Rowlandson, G.I. Improved pacemaker pulse detection: Clinical evaluation of a new high-bandwidth electrocardiographic system. J. Electrocardiol. 2011, 44, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Johnston, P.; Macfarlane, P.W. Implanted cardiac pacemaker pulses as recorded from the body surface. J. Electrocardiol. 2012, 45, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Ricke, A.D.; Swiryn, S.; Sahakian, A.V.; Petrutiu, S.; Young, B.; Rowlandson, G.I. The relationship between programmed pacemaker pulse amplitude and he surface electrocardiogram recorded amplitude: Application of a new high-bandwidth electrocardiogram system. J. Electrocardiol. 2008, 41, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.; Sahakian, A.V.; Ricke, A.; Swiryn, S. Observations of pacemaker pulses in high-bandwidth electrocardiograms and Dower-estimated vectorcardiograms. J. Electrocardiol. 2011, 44, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, P.; Gargiulo, G.D.; Romano, M.; Cesarelli, M. A simple, wide bandwidth, biopotential amplifier to record pacemaker pulse waveform. Med. Devices 2016, 9, 325–329. [Google Scholar]
- Jacob, S.; Panaich, S.S.; Maheshwari, R.; Haddad, J.W.; Padanilam, B.J.; John, S.K. Clinical application of magnets on cardiac rhythm management devices. Europace 2011, 13, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, P.; Cesarelli, M.; D’Apuzzo, M.; Gargiulo, G.D.; Liccardo, A.; Pasquino, N.; Romano, M.; Moriello, R.S.L. A Low-Cost Device for Contactless Detection of Pacemaker Pulses. Int. Rev. Electr. Eng. 2013, 8, 1461–1466. [Google Scholar]
- Pethig, R. Dielectric properties of body tissues. Clin. Phys. Physiol. Meas. 1987, 8, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Du, M.; Qu, L.; Chen, S.; Zhuabc, S.; Hanbc, G. Electromagnetic interference shielding cotton fabrics with high electrical conductivity and electrical heating behavior via layer-by-layer self- assembly route. RSC Adv. 2017, 7, 42641–42652. [Google Scholar] [CrossRef]
- Garcia, M.A.; Pinel, E.F.; de la Venta, J.; Quesada, A.; Bouzas, V.; Fernández, J.F.; Romero, J.J.; González, M.S.M.; Costa-Krämer, J.L. Sources of experimental errors in the observation of nanoscale magnetism. J. Appl. Phys. 2009, 105, 013925. [Google Scholar] [CrossRef] [Green Version]
- Ehrmann, A.; Blachowicz, T. Examination of Textiles with Mathematical and Physical Methods; Springer International Publishing: New York, NY, USA, 2017; Volume 3, pp. 40–42. ISBN 978-3-319-47406-9. [Google Scholar]
- Chudasama, D. Protection against electromagnetic radiation with textile material. Int. J. Electr. Electron. Eng. Res. 2013, 3, 293–302. [Google Scholar]
- ISO 14117:2012. Active Implantable Medical Devices. Electromagnetic Compatibility. EMC Test Protocols for Implantable Cardiac Pacemakers, Implantable Cardioverter Defibrillators and Cardiac Resynchronization Devices. Available online: https://www.iso.org/standard/54472.html (accessed on 16 March 2018).
- St. Jude Medical Manuals. Available online: https://manuals.sjm.com/~/media/manuals/product-manual-pdfs/c/c/ccf383b9-f91a-424f-bd8f-dd4472991fca.pdf (accessed on 16 March 2018).
- Turin, G.L. An introduction to digital matched filters. Proc. IEEE 1976, 64, 1092–1112. [Google Scholar] [CrossRef]
- ISO 8601:2004. Data Elements and Interchange Formats—Information Interchange—Representation of Dates and Times. Available online: https://www.iso.org/iso-8601-date-and-time-format.html (accessed on 16 March 2018).
- Gargiulo, G.; Bifulco, P.; Cesarelli, M.; Jin, C.; McEwan, A.; van Schaik, A. Wearable dry sensors with bluetooth connection for use in remote patient monitoring systems. Stud. Health Technol. Inform. 2010, 161, 57–65. [Google Scholar] [PubMed]













| Parameter | Value |
|---|---|
| µr | 155 |
| N | 56 |
| S | 0.096 cm2 |
| VSTIM | 7.5 V |
| Rel | 640 Ω |
| tRISE | 8 µs |
| cos γ | 1 |
| G | 1013 |
| Ar | 1.561 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreozzi, E.; Gargiulo, G.D.; Fratini, A.; Esposito, D.; Bifulco, P. A Contactless Sensor for Pacemaker Pulse Detection: Design Hints and Performance Assessment. Sensors 2018, 18, 2715. https://doi.org/10.3390/s18082715
Andreozzi E, Gargiulo GD, Fratini A, Esposito D, Bifulco P. A Contactless Sensor for Pacemaker Pulse Detection: Design Hints and Performance Assessment. Sensors. 2018; 18(8):2715. https://doi.org/10.3390/s18082715
Chicago/Turabian StyleAndreozzi, Emilio, Gaetano D. Gargiulo, Antonio Fratini, Daniele Esposito, and Paolo Bifulco. 2018. "A Contactless Sensor for Pacemaker Pulse Detection: Design Hints and Performance Assessment" Sensors 18, no. 8: 2715. https://doi.org/10.3390/s18082715
APA StyleAndreozzi, E., Gargiulo, G. D., Fratini, A., Esposito, D., & Bifulco, P. (2018). A Contactless Sensor for Pacemaker Pulse Detection: Design Hints and Performance Assessment. Sensors, 18(8), 2715. https://doi.org/10.3390/s18082715