Simultaneous Voltammetric Determination of Acetaminophen, Ascorbic Acid and Uric Acid by Use of Integrated Array of Screen-Printed Electrodes and Chemometric Tools
Abstract
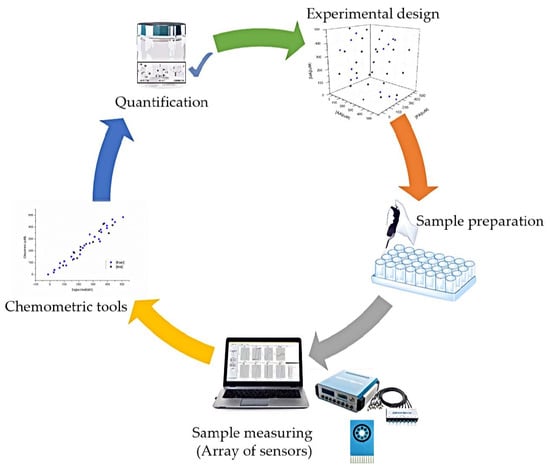
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Electronic Tongue
2.3. Modification of the Electrode Surface
2.4. Characterization by Scanning Electron Microscopy
2.5. Sample Preparation
2.6. Data Processing
3. Results and Discussion
3.1. Characterization of the Surface
3.2. Voltammetric Array Response
3.3. Characterization of the Modified Integrated Screen-Printed Electrodes
3.3.1. Calibration Curves
3.3.2. Stability and Reproducibility Studies
3.4. Qualitative Analysis: Principal Component Analysis (PCA)
3.5. Quantitative Analysis: Partial Least Squares (PLS) Regression
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanghavi, B.J.; Srivastava, A.K. Simultaneous voltammetric determination of acetaminophen, aspirin and caffeine using an in situ surfactant-modified multiwalled carbon nanotube paste electrode. Electrochim. Acta 2010, 55, 8638–8648. [Google Scholar] [CrossRef]
- Madrakian, T.; Haghshenas, E.; Afkhami, A. Simultaneous determination of tyrosine, acetaminophen and ascorbic acid using gold nanoparticles/multiwalled carbon nanotube/glassy carbon electrode by differential pulse voltammetric method. Sens. Actuators B Chem. 2014, 193, 451–460. [Google Scholar] [CrossRef]
- Hu, G.; Ma, Y.; Guo, Y.; Shao, S. Electrocatalytic oxidation and simultaneous determination of uric acid and ascorbic acid on the gold nanopartides-modified glassy carbon electrode. Electrochim. Acta 2008, 53, 6610–6615. [Google Scholar] [CrossRef]
- Beard, E.L. The American society of health system pharmacists. JONA’s Healthc. Law Eth. Regul. 2001, 3, 78–79. [Google Scholar] [CrossRef]
- Kaur, H.; Halliwell, B. Action of biologically-relevant oxidizing species upon uric acid. Identification of uric acid oxidation products. Chem. Biol. Interact. 1990, 73, 235–247. [Google Scholar] [CrossRef]
- Miland, E.; Miranda Ordieres, A.J.; Tuñón Blanco, P.; Smyth, M.R.; Fágáin, C.Ó. Poly(o-Aminophenol)-Modified Bienzyme Carbon Paste Electrode for the Detection of Uric Acid. Talanta 1996, 43, 785–796. [Google Scholar] [CrossRef]
- Dejaegher, B.; Bloomfield, M.S.; Smeyers-Verbeke, J.; Vander Heyden, Y. Validation of a Fluorimetric assay for 4-aminophenol in paracetamol formulations. Talanta 2008, 75, 258–265. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Šatínský, D.; Solich, P.; Ortega-Barrales, P.; Molina-Díaz, A. Fluorimetric SIA optosensing in pharmaceutical analysis: Determination of paracetamol. J. Pharm. Biomed. Anal. 2007, 45, 318–321. [Google Scholar] [CrossRef]
- Sirajuddin; Khaskheli, A.R.; Shah, A.; Bhanger, M.I.; Niaz, A.; Mahesar, S. Simpler spectrophotometric assay of paracetamol in tablets and urine samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 747–751. [Google Scholar] [CrossRef]
- Ortega Barrales, P.; Fernández De Córdova, M.L.; Molina Díaz, A. Indirect determination of ascorbic acid by solid-phase spectrophotometry. Anal. Chim. Acta 1998, 360, 143–152. [Google Scholar] [CrossRef]
- Akay, C.; Gümüsel, B.; Degim, T.; Tartilmis, S.; Cevheroglu, S. Simultaneous determination of acetaminophen, acetylsalicylic acid and ascorbic acid in tablet form using HPLC. Drug Metabol. Drug Interact. 1999, 15, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Thomis, R.; Roets, E.; Hoogmartens, J. Analysis of tablets containing aspirin, acetaminophen, and ascorbic acid by high-performance liquid chromatography. J. Pharm. Sci. 1984, 73, 1830–1833. [Google Scholar] [CrossRef] [PubMed]
- Capella-Peiró, M.E.; Bose, D.; Rubert, M.F.; Esteve-Romero, J. Optimization of a capillary zone electrophoresis method by using a central composite factorial design for the determination of codeine and paracetamol in pharmaceuticals. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 839, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Niu, Y.; Bi, S.; Zhang, S. Determination of ascorbic acid in individual rat hepatocyte cells based on capillary electrophoresis with electrochemiluminescence detection. Electrophoresis 2008, 29, 2918–2924. [Google Scholar] [CrossRef] [PubMed]
- Švorc, L. Determination of caffeine: A comprehensive review on electrochemical methods. Int. J. Electrochem. Sci. 2013, 8, 5755–5773. [Google Scholar]
- Zhu, X.; Xu, J.; Duan, X.; Lu, L.; Zhang, K.; Yu, Y.; Xing, H.; Gao, Y.; Dong, L.; Sun, H.; et al. Controlled synthesis of partially reduced graphene oxide: Enhance electrochemical determination of isoniazid with high sensitivity and stability. J. Electroanal. Chem. 2015, 757, 183–191. [Google Scholar] [CrossRef]
- Ren, W.; Luo, H.Q.; Li, N.B. Simultaneous voltammetric measurement of ascorbic acid, epinephrine and uric acid at a glassy carbon electrode modified with caffeic acid. Biosens. Bioelectron. 2006, 21, 1086–1092. [Google Scholar] [CrossRef]
- Bard, A.J. Chemical modification of electrodes. J. Chem. Educ. 1983, 60, 302. [Google Scholar] [CrossRef]
- Serrano, N.; Prieto-Simón, B.; Cetó, X.; del Valle, M. Array of peptide-modified electrodes for the simultaneous determination of Pb(II), Cd(II) and Zn(II). Talanta 2014, 125, 159–166. [Google Scholar] [CrossRef]
- Serrano, N.; González-Calabuig, A.; del Valle, M. Crown ether-modified electrodes for the simultaneous stripping voltammetric determination of Cd(II), Pb(II) and Cu(II). Talanta 2015, 138, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Penicillamine-modified sensor for the voltammetric determination of Cd(II) and Pb(II) ions in natural samples. Talanta 2015, 144, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wawrzyniak, U.E.; Ciosek, P.; Zaborowski, M.; Liu, G.; Gooding, J.J. Gly-Gly-His immobilized on monolayer modified back-side contact miniaturized sensors for complexation of copper ions. Electroanalysis 2013, 25, 1461–1471. [Google Scholar] [CrossRef]
- Cipri, A.; del Valle, M. Pd nanoparticles/multiwalled carbon nanotubes electrode system for voltammetric sensing of tyrosine. J. Nanosci. Nanotechnol. 2014, 14, 6692–6698. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, M. Bioinspired sensor systems. Sensors 2011, 11, 10180–10186. [Google Scholar] [CrossRef] [PubMed]
- Toko, K. Biomimetic Sensor Technology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Röck, F.; Barsan, N.; Weimar, U. Electronic nose: Current Status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Toko, K. Electronic tongues—A review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Pallejà, T.; Tresanchez, M.; Teixidó, M.; Palacin, J. Bioinspired electronic white cane implementation based on a LIDAR, a Tri-Axial accelerometer and a tactile belt. Sensors 2010, 10, 11322–11339. [Google Scholar] [CrossRef] [PubMed]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; di Natale, C.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids (IUPAC technical report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Lavine, B.K.; Workman, J., Jr. Chemometrics. Anal. Chem. 2002, 74, 2763–2769. [Google Scholar] [CrossRef]
- Hanrahan, G. Computational neural networks driving complex analytical problem solving. Anal. Chem. 2010, 82, 4307–4313. [Google Scholar] [CrossRef]
- Dropsens. 8W Screen-Printed Carbon Electrode. Available online: http://www.dropsens.com/ pdfs_productos/new_brochures/8w110.pdf (accessed on 25 July 2019).
- Daniels, J.S.; Pourmand, N. Label-free impedance biosensors: Opportunities and challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- González-Sánchez, M.I.; Gómez-Monedero, B.; Agrisuelas, J.; Iniesta, J.; Valero, E. Highly activated screen-printed carbon electrodes by electrochemical treatment with hydrogen peroxide. Electrochem. Commun. 2018, 91, 36–40. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 8th ed.; Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2013. [Google Scholar]
- Cetó, X.; Céspedes, F.; Pividori, M.I.; Gutiérrez, J.M.; del Valle, M. Resolution of phenolic antioxidant mixtures employing a voltammetric bio-electronic tongue. Analyst 2012, 137, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Alegret, S.; Alonso, J.; Bartrolí, J.; Céspedes, F.; Martínez-Fàbregas, E.; del Valle, M. Amperometric Biosensors based on bulk-modified epoxy graphite biocomposites. Sens. Mater. 1996, 8, 147–153. [Google Scholar]
- Gemperline, P. Practical Guide to Chemometrics, 2nd ed.; CRC press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Abdi, H. Partial least squares regression and projection on latent structure regression (PLS regression). Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Gutés, A.; Calvo, D.; Céspedes, F.; del Valle, M. Automatic sequential injection analysis electronic tongue with integrated reference electrode for the determination of ascorbic acid, uric acid and paracetamol. Microchim. Acta 2007, 157, 1–6. [Google Scholar] [CrossRef]










| Compounds | Graphite | Cobalt (II) Phthalocyanine | Copper Oxide (II) | Prussian Blue |
|---|---|---|---|---|
| Acetaminophen | y = 0.1234x + 2.6656 R2 = 0.993 | y = 0.1057x + 2.7753 R2 = 0.991 | y = 0.1749x − 4.0453 R2 = 0.997 | y = 0.1696x + 7.5897 R2 = 0.984 |
| Ascorbic Acid | y = 0.0398x + 6.5307 R2 = 0.997 | y = 0.0311x + 8.5678 R2 = 0.993 | y = 0.0372x + 3.8868 R2 = 0.992 | y = 0.0214x + 9.0457 R2 = 0.992 |
| Uric Acid | y = 0.1298x + 4.6275 R2 = 0.999 | y = 0.0534x + 9.3828 R2 = 0.996 | y = 0.1569x + 15.231 R2 = 0.988 | y = 0.1074x + 7.6534 R2 = 0.995 |
| Sensor | RSD% |
|---|---|
| Graphite/SPCE-Ink | 2.9 |
| Cobalt (II) phthalocyanine/SPCE-Ink | 7.5 |
| Copper oxide (II)/SPCE-Ink | 1.3 |
| Prussian blue/SPCE-Ink | 0.8 |
| Set | Analyte | R2 | Slope | Intercept (µmol·L−1) | NRMSE |
|---|---|---|---|---|---|
| Acetaminophen | 0.962 | 1.00 ± 0.09 | 0 ± 25 | 0.90 | |
| Training Set | Ascorbic acid | 0.955 | 1.00 ± 0.09 | 0 ± 25 | 0.97 |
| (n = 27) | Uric acid | 0.940 | 1.00 ± 0.11 | 0 ± 31 | 1.12 |
| Acetaminophen | 0.915 | 1.02 ± 0.22 | −13 ± 28 | 0.7 | |
| Testing Set | Ascorbic acid | 0.762 | 1.07 ± 0.42 | −3 ± 54 | 1.41 |
| (n = 12) | Uric acid | 0.829 | 1.04 ± 0.33 | −32 ± 36 | 0.85 |
| Set | Analyte | R2 | Slope | Intercept (µmol·L−1) |
|---|---|---|---|---|
| Acetaminophen | 0.968 | 0.942 ± 0.031 | 32 ± 21 | |
| Training Set | Ascorbic acid | 0.947 | 0.933 ± 0.040 | 36 ± 25 |
| (n = 33) | Uric acid | 0.923 | 0.873 ± 0.046 | 58 ± 25 |
| Acetaminophen | 0.848 | 0.895 ± 0.105 | 82 ± 71 | |
| Testing Set | Ascorbic acid | 0.908 | 0.919 ± 0.081 | 65 ± 41 |
| (n = 15) | Uric acid | 0.753 | 0.871 ± 0.138 | −8 ± 86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Aguayo, D.; Bonet-San-Emeterio, M.; del Valle, M. Simultaneous Voltammetric Determination of Acetaminophen, Ascorbic Acid and Uric Acid by Use of Integrated Array of Screen-Printed Electrodes and Chemometric Tools. Sensors 2019, 19, 3286. https://doi.org/10.3390/s19153286
Ortiz-Aguayo D, Bonet-San-Emeterio M, del Valle M. Simultaneous Voltammetric Determination of Acetaminophen, Ascorbic Acid and Uric Acid by Use of Integrated Array of Screen-Printed Electrodes and Chemometric Tools. Sensors. 2019; 19(15):3286. https://doi.org/10.3390/s19153286
Chicago/Turabian StyleOrtiz-Aguayo, Dionisia, Marta Bonet-San-Emeterio, and Manel del Valle. 2019. "Simultaneous Voltammetric Determination of Acetaminophen, Ascorbic Acid and Uric Acid by Use of Integrated Array of Screen-Printed Electrodes and Chemometric Tools" Sensors 19, no. 15: 3286. https://doi.org/10.3390/s19153286
APA StyleOrtiz-Aguayo, D., Bonet-San-Emeterio, M., & del Valle, M. (2019). Simultaneous Voltammetric Determination of Acetaminophen, Ascorbic Acid and Uric Acid by Use of Integrated Array of Screen-Printed Electrodes and Chemometric Tools. Sensors, 19(15), 3286. https://doi.org/10.3390/s19153286