Molecular-Charge-Contact-Based Ion-Sensitive Field-Effect Transistor Sensor in Microfluidic System for Protein Sensing
Abstract
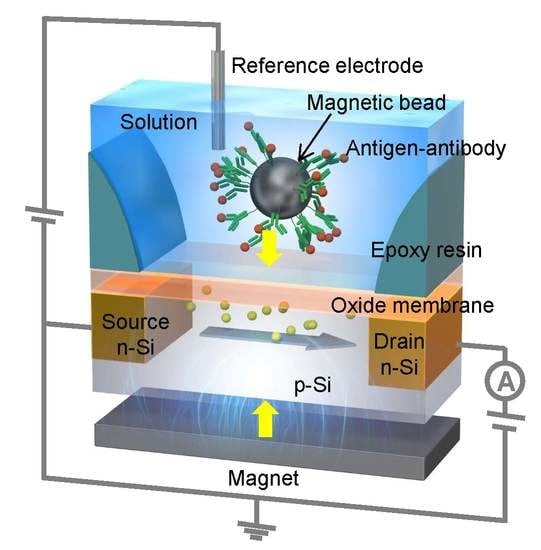
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Pretreatment of Magnetic Beads
2.3. Detection of Streptavidin by MCC Method with Microfluidic System
3. Results and Discussion
3.1. Potentiometric Detection of Streptavidin Using MCC Method in Microfluidic System
3.2. Limit of Detection
3.3. Detection Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergveld, P. Development of an Ion-Sensitive Solid-State Device for Neurophysiological Measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar] [CrossRef]
- Bergveld, P. Development, Operation, and Application of the Tool for Electrophysiology. IEEE Trans. Biomed. Eng. 1972, BME-19, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Wise, K.D. An Integrated Field-Effect Electrode for Biopotential Recording. IEEE Trans. Biomed. Eng. 1974, BME-21, 485–487. [Google Scholar] [CrossRef]
- Esashi, M.; Matsuo, T. Integrated Micro-Multi-Ion Sensor Using Field Effect of Semiconductor. IEEE Trans. Biomed. Eng. 1978, BME-25, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Schenck, J.F. Technical Difficulties Remaining to the Application of ISFET Devices, Theory Design and Biomedical Applications of Solid State Chemical Sensors; Cheung, P.W., Ed.; CRC Press: Boca Raton, FL, USA, 1978; pp. 165–173. [Google Scholar]
- Stern, H.O. Zur Theorie der Elektrolytischen Doppelschicht. Zeitschrift für Elektrochemie und Angewandte Physikalische Chemie 1924, 30, 508–516. [Google Scholar] [CrossRef]
- Schasfoort, R.B.M.; Kooyman, R.P.H.; Bergveld, P.; Greve, J. A New Approach to ImmunoFET Operation. Biosens. Bioelectron. 1990, 5, 103–124. [Google Scholar] [CrossRef]
- Schasfoort, R.B.M.; Bergveld, P.; Kooyman, R.P.H.; Greve, J. Possibilities and Limitations of Direct Detection of Protein Charges by Means of an Immunological Field-Effect Transistor. Anal. Chim. Acta 1990, 238, 323–329. [Google Scholar] [CrossRef]
- Bergveld, P. A Critical Evaluation of Direct Electrical Protein Detection Methods. Biosens. Bioelectron. 1991, 6, 55–72. [Google Scholar] [CrossRef]
- Souteyrand, E.; Cloarec, J.P.; Martin, J.R.; Wilson, C.; Lawrence, I.; Mikkelsen, S.; Lawrence, M.F. Direct Detection of the Hybridization of Synthetic Homo-Oligomer DNA Sequences by Field Effect. J. Phys. Chem. B 1997, 101, 2980–2985. [Google Scholar] [CrossRef]
- Berney, H.; West, J.; Haefele, E.; Alderman, J.; Lane, W.; Collins, J.K. A DNA Diagnostic Biosensor: Development, Characterisation and Performance. Sens. Actuators B Chem. 2000, 68, 100–108. [Google Scholar] [CrossRef]
- Fritz, J.; Cooper, E.B.; Gaudet, S.; Sorger, P.K.; Manalis, S.R. Electronic Detection of DNA by Its Intrinsic Molecular Charge. Proc. Natl. Acad. Sci. USA 2002, 99, 14142–14146. [Google Scholar] [CrossRef] [PubMed]
- Schöning, M.J.; Poghossian, A. Recent Advances in Biologically Sensitive Field-Effect Transistors (BioFETs). Analyst 2002, 127, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Uslu, F.; Ingebrandt, S.; Mayer, D.; Böcker-Meffert, S.; Odenthal, M.; Offenhäusser, A. Labelfree Fully Electronic Nucleic Acid Detection System Based on a Field-Effect Transistor Device. Biosens. Bioelectron. 2004, 19, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Miyahara, Y. Potentiometric Detection of Single Nucleotide Polymorphism Using Genetic Field Effect Transistor. ChemBioChem 2005, 6, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Miyahara, Y. DNA Sequencing Based on Intrinsic Molecular Charges. Angew. Chem. Int. Ed. 2006, 45, 2225–2228. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Miyahara, Y. Direct Transduction of Primer Extension into Electrical Signal Using Genetic Field Effect Transistor. Biosens. Bioelectron. 2007, 22, 1311–1316. [Google Scholar] [CrossRef]
- Ingebrandt, S.; Han, Y.; Nakamura, F.; Poghossian, A.; Schöning, M.J.; Offenhäusser, A. Label-Free Detection of Single Nucleotide Polymorphisms Utilizing the Differential Transfer Function of Field-Effect Transistors. Biosens. Bioelectron. 2007, 22, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, Y.; Sakata, T. Molecular Charge Contact Biosensing Based on the Interaction of Biologically Modified Magnetic Beads with an Ion-Sensitive Field Effect Transistor. Eur. Biophys. J. 2014, 43, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Satake, H.; Saito, A.; Sakata, T. Elucidation of interfacial pH behaviour at cell/substrate nanogap for in situ monitoring of cellular respiration. Nanoscale 2018, 10, 10130–10136. [Google Scholar] [CrossRef]
- Sakata, T.; Saito, A.; Sugimoto, H. Live Monitoring of Microenvironmental pH Based on Extracellular Acidosis around Cancer Cells with Cell-Coupled Gate Ion-Sensitive Field-Effect Transistor. Anal. Chem. 2018, 90, 12731–12736. [Google Scholar] [CrossRef]
- Debye, P.; Hückel, E. Zur Theorie der Elektrolyte. Physikalische Zeitschrift 1923, 24, 185–206. [Google Scholar]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-Free Immunodetection with CMOS-Compatible Semiconducting Nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Wagner, R.; Sigworth, F.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the Debye Screening Length on Nanowire Field Effect Transistor Sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, H. Die berechnung der nachweisempfindlichkeit. Spectrochim. Acta 1947, 3, 40–67. [Google Scholar] [CrossRef]
- Gebauer, C.R.; Rechnitz, G.A. Ion Selective Electrode Estimation of Avidin and Biotin Using a Lysozyme Label. Anal. Biochem. 1980, 103, 280–284. [Google Scholar] [CrossRef]
- Mock, D.M.; Langford, G.; Dubois, D.; Criscimagna, N.; Horowitz, P. A Fluorometric Assay for the Biotin-Avidin Interaction Based on Displacement of the Fluorescent Probe 2-Anilinonaphthalene-6-sulfonic Acid. Anal. Biochem. 1985, 151, 178–181. [Google Scholar] [CrossRef]
- Schray, K.J.; Artz, P.G.; Hevey, R.C. Determination of Avidin and Biotin by Fluorescence Polarization. Anal. Chem. 1988, 60, 853–855. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, H.; Gu, D.; He, J. A Quartz Crystal Microbalance as a Tool for Biomolecular Interaction Studies. RSC Adv. 2015, 5, 64520–64525. [Google Scholar] [CrossRef]
- Focsan, M.; Campu, A.; Craciun, A.-M.; Potara, M.; Leordean, C.; Maniu, D.; Astilean, S. A Simple and Efficient Design to Improve the Detection of Biotin-Streptavidin Interaction with Plasmonic Nanobiosensors. Biosens. Bioelectron. 2016, 86, 728–735. [Google Scholar] [CrossRef]
- Yang, H.; Honda, M.; Akiko, A.; Kajisa, T.; Yanase, Y.; Sakata, T. Nonoptical Detection of Allergic Response with a Cell-Coupled Gate Field-Effect Transistor. Anal. Chem. 2017, 89, 12918–12923. [Google Scholar] [CrossRef]
- Saito, A.; Sakata, T. Sperm-Cultured Gate Ion-Sensitive Field-Effect Transistor for Nonoptical and Live Monitoring of Sperm Capacitation. Sensors 2019, 19, 1784. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Sakata, T. Molecular-Charge-Contact-Based Ion-Sensitive Field-Effect Transistor Sensor in Microfluidic System for Protein Sensing. Sensors 2019, 19, 3393. https://doi.org/10.3390/s19153393
Yang H, Sakata T. Molecular-Charge-Contact-Based Ion-Sensitive Field-Effect Transistor Sensor in Microfluidic System for Protein Sensing. Sensors. 2019; 19(15):3393. https://doi.org/10.3390/s19153393
Chicago/Turabian StyleYang, Haoyue, and Toshiya Sakata. 2019. "Molecular-Charge-Contact-Based Ion-Sensitive Field-Effect Transistor Sensor in Microfluidic System for Protein Sensing" Sensors 19, no. 15: 3393. https://doi.org/10.3390/s19153393
APA StyleYang, H., & Sakata, T. (2019). Molecular-Charge-Contact-Based Ion-Sensitive Field-Effect Transistor Sensor in Microfluidic System for Protein Sensing. Sensors, 19(15), 3393. https://doi.org/10.3390/s19153393