A Nanoparticle-Based Label-Free Sensor for Screening the Relative Antioxidant Capacity of Hydrosoluble Plant Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Plant Extracts
2.3. Instrumentation
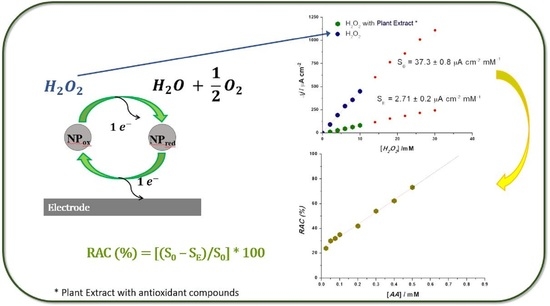
3. Results and Discussions
3.1. Electrochemical Sensor Characterization and Optimization
3.2. Antioxidant Properties of the Plant Extracts
3.2.1. Scanning Electron Microscopy
3.2.2. Electrochemical Evaluation of Relative Antioxidant Capacity
Cyclic Voltammetry
Amperometry-H2O2 Sensor for Relative Antioxidant Capacity
3.2.3. Statistical Analysis
Spectrophotometry
Chemiluminescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Zuo, T.; Zhu, M.; Xu, W. Roles of Oxidative Stress in Polycystic Ovary Syndrome and Cancers. Oxid. Med. Cell. Longev. 2016, 2016, 8589318. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food. Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Périno-Issartier, S.; Zill-e-Huma; Abert-Vian, M.; Chemat, F. Solvent Free Microwave-Assisted Extraction of Antioxidants from Sea Buckthorn (Hippophae rhamnoides) Food By-Products. Food Bioprocess Technol. 2011, 4, 1020–1028. [Google Scholar] [CrossRef]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [Green Version]
- Komes, D.; Belščak-Cvitanović, A.; Horžić, D.; Rusak, G.; Likić, S.; Berendika, M. Phenolic Composition and Antioxidant Properties of Some Traditionally Used Medicinal Plants Affected by the Extraction Time and Hydrolysis. Phytochem. Anal. 2011, 122, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.; Madureira, A.M.; Jorge, R.; Adão, R.; Duarte, A.; Duarte, N.; Lopes, M.M.; Teixeira, G. Antioxidant and Antimycotic Activities of Two Native Lavandula Species from Portugal. Evid. Based Complement. Altern. Med. 2015, 2015, 570521. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Masa, A.; Vilanova, M.; Pomar, F. Varietal differences among the flavonoid profiles of white grape cultivars studied by high-performance liquid chromatography. J. Chromatogr. A 2007, 1164, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.F.R.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Zargoosh, K.; Ghayeb, Y.; Aeineh, N.; Qandalee, M. Evaluation of Antioxidant Capacity of Hydrophilic and Hydrophobic Antioxidants Using Peroxyoxalate Chemiluminescence Reaction of the Novel Furandicarboxylate Derivative. Food Anal. Methods 2014, 7, 283–290. [Google Scholar] [CrossRef]
- Guliyev, V.B.; Gul, M.; Yildirim, A. Hippophae rhamnoides L.: Chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. J. Chromatogr. B 2004, 812, 291–307. [Google Scholar] [CrossRef]
- Moţ, A.C.; Silaghi-Dumitrescu, R.; Sârbu, C. Rapid and Effective Evaluation of the Antioxidant Capacity of Propolis Extracts Using DPPH Bleaching Kinetic Profiles; FT-IR and UV-vis Spectroscopic Data. J. Food Compos. Anal. 2011, 24, 516–522. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Varzakova, D.P.; Gerasimova, E.L. A chronoamperometric method for determining total antioxidant activity. J. Anal. Chem. 2012, 67, 364–369. [Google Scholar] [CrossRef]
- Bucur, M.P.; Rădulescu, M.C.; Bucur, B.; Radu, G.L. Low-interferences Determination of the Antioxidant Capacity in Fruits Juices Based on Xanthine Oxidase and Mediated Amperometric Measurements in the Reduction Mode. Anal. Sci. 2016, 32, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Ge, Q.; Ge, P.; Jiang, D.; Du, N.; Chen, J.; Yuan, L.; Yu, H.; Xu, X.; Wu, M.; Zhang, W.; et al. A novel and simple cell-based electrochemical biosensor for evaluating the antioxidant capacity of Lactobacillus plantarum strains isolated from Chinese dry-cured ham. Biosens. Bioelectron. 2018, 99, 555–563. [Google Scholar] [CrossRef]
- David, M.; Bârsan, M.M.; Florescu, M.; Brett, C.M.A. Acidic and Basic Functionalized Carbon Nanomaterials as Electrical Bridges in Enzyme Loaded Chitosan/ Poly(styrene sulfonate) Self-Assembled Layer-by-Layer Glucose Biosensors. Electroanalysis 2015, 27, 2139–2149. [Google Scholar] [CrossRef]
- Annapandian, V.M.; Rajagopal, S.S. Phytochemical Evaluation and In vitro Antioxidant Activity of Various Solvent Extracts of Leucas aspera (Willd.) Link Leaves. Free Radic. Antioxid. 2017, 7, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Brownson, D.A.C.; Banks, C.E. Graphene electrochemistry: An overview of potential applications. Analyst 2010, 135, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Memana, N.M.; Pourkhalil, M.; Rashidi, A.; Nezhad, B.Z. Synthesis, characterization and operation of a functionalized multi-walled CNT supported MnOx nanocatalyst for deep oxidative desulfurization of sour petroleum fractions. J. Ind. Eng. Chem. 2014, 20, 4054–4058. [Google Scholar] [CrossRef]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble Metal Nanoparticles for Biosensing Applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Chu, L.; Liu, Y.; Wang, A.; Ji, B.; Wu, W.; Zhou, F.; Wei, Y.; Cheng, Q.; Cai, S.; et al. Analysis of the Antioxidant Capacities of Flavonoids under Different Spectrophotometric Assays Using Cyclic Voltammetry and Density Functional Theory. J. Agric. Food Chem. 2011, 59, 10277–10285. [Google Scholar] [CrossRef]
- Sochor, J.; Dobes, J.; Krystofova, O.; Ruttkay-Nedecky, B.; Babula, P.; Pohanka, M.; Jurikova, T.; Zitka, O.; Adam, V.; Klejdus, B.; et al. Electrochemistry as a tool for studying antioxidant properties. Int. J. Electrochem. Sci. 2013, 8, 8464–8489. [Google Scholar]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [Green Version]
- Rădulescu, C.; Stihi, C.; Ilie, M.; Lazurcă, D.; Gruia, R.; Olaru, O.T.; Bute, O.C.; Dulama, I.D.; Ştirbescu, R.M.; Teodorescu, S.; et al. Characterization of Phenolics in Lavandula angustifolia. Anal. Lett. 2017, 50, 2839–2850. [Google Scholar] [CrossRef]
- Piljac-Žegarac, J.; Valek, L.; Stipčević, T.; Martinez, S. Electrochemical determination of antioxidant capacity of fruit tea infusions. Food Chem. 2010, 121, 820–825. [Google Scholar] [CrossRef]
- Campanella, L.; Martini, E.; Tomassetti, M. Antioxidant capacity of the algae using a biosensor method. Talanta 2005, 66, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Arbeláez, J.; Vázqueza, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska-Świgło, A. Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem. 2006, 96, 131–136. [Google Scholar] [CrossRef]
- Oktay, M.; Gülҫin, I.; Küfrevioğlu, Ö.I. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT Food Sci. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Karyakina, E.E.; Vokhmyanina, D.V.; Sizova, N.V.; Sabitov, A.N.; Borisova, A.V.; Sazontova, T.G.; Arkhipenko, Y.V.; Tkachuk, V.A.; Zolotov, Y.A.; Karyakin, A.A. Kinetic approach for evaluation of total antioxidant activity. Talanta 2009, 80, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Bunaciu, A.A.; Dăneţ, A.F.; Fleschin, Ş.; Aboul-Enein, H.Y. Recent applications for in vitro antioxidant assay. Crit. Rev. Anal. Chem. 2016, 46, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lo, Y.R.; Lin, Y.C.; Shi, Y.C.; Li, P.L. A Spectrometric Method for Hydrogen Peroxide Concentration Measurement with a Reusable and Cost-Efficient Sensor. Sensors 2015, 15, 25716–25729. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 4th ed.; Pearson Education Limited: Harlow, UK, 2000; pp. 48–50, Appendix 2; ISBN 0 130 22888 5. [Google Scholar]
- Popa, C.V.; Lungu, L.; Savoiu, M.; Bradu, C.; Dinoiu, V.; Dăneţ, A.F. Total Antioxidant Activity and Phenols and Flavonoids Content of Several Plant Extracts. Int. J. Food Prop. 2012, 15, 691–701. [Google Scholar] [CrossRef]












| Solvent Type | Hippophaefructus Extracts | Lavandula Flowers Extracts |
|---|---|---|
| Hydroalcoholic solution (1:1) water “a”: ethanol | 1.Hf Aa T / 1.Hf Aa US | 1.Lf Aa T / 1.Lf Aa US |
| Hydroalcoholic solution (1:1) water “b”: ethanol | 2.Hf Ab T / 2.Hf AbUS | 2.Lf Ab T / 2.Lf Ab US |
| Hydroglycerine solution (1:1) water “a”: glycerin | 3.Hf Ga T / 3.Hf Ga US | 3.Lf Ga T / 3.Lf Ga US |
| Hydroglycerine solution (1:1) water “b”: glycerin | 4.Hf Gb T / 4.Hf Gb US | 4.Lf Gb T / 4.Lf Gb US |
| Hydropropyleneglycol solution (1:1) water “a”: propylene glycol | 5.Hf Pga T / 5.Hf Pga US | 5.Lf Pga T / 5.Lf Pga US |
| Hydropropyleneglycol solution (1:1) water “b”: propylene glycol | 6.Hf Pgb T / 6.Hf Pgb US | 6.Lf Pgb T / 6.Lf Pgb US |
| Solvent Type | Juglans Regia Extracts | Vitis ViniferaExtracts |
|---|---|---|
| Hydroalcoholic solution (1:1) water “a”: ethanol | 1.Js Aa T / 1.Js Aa US | 1.Vp Aa T / 1.Vp Aa US |
| Extracts | Electrochemical RAC (%) | Spectrophotometry RAC (%) |
|---|---|---|
| 1.Lf Aa US | 83.8 ± 0.04 | 77.6 ± 0.11 |
| 1.Hf Aa US | 72.8 ± 0.22 | 79.0 ± 0.09 |
| 1.Js Aa US | 30.9 ± 0.32 | 56.7 ± 0.92 |
| 1.Vp Aa US | 47.6 ± 0.83 | 73.4 ± 1.08 |
| 1.Lf Aa T | 78.5 ± 0.03 | 84.5 ± 0.07 |
| 1.Hf Aa T | 69.6 ± 0.20 | 81.9 ± 0.24 |
| 1.Js Aa T | 20.0 ± 0.44 | 33.3 ± 1.05 |
| 1.Vp Aa T | 36.7 ± 0.23 | 58.2 ± 2.11 |
| Extracts | Electrochemistry TE (mg/100 mL extract) | Chemiluminescence TE (mg/100 mL extract) |
|---|---|---|
| 1.Hf Aa US | 45.1 ± 0.22 | 49.2 ± 0.90 |
| 2.Hf Ab T | 42.0 ± 0.85 | 42.5 ± 1.40 |
| 3.Hf Ga T | 22.0 ± 0.90 | 29.4 ± 1.00 |
| 3.Hf Ga US | 47.8 ± 0.36 | 44.6 ± 0.82 |
| 4.Hf Gb US | 53.5 ± 1.28 | 44.8 ± 3.90 |
| 5.Hf PGa T | 42.3 ± 0.78 | 39.9 ± 1.50 |
| 5.Hf PGa US | 48.4 ± 0.36 | 48.1 ± 0.80 |
| 6.Hf PGb T | 35.0 ± 0.60 | 40.7 ± 1.50 |
| 3.Lf Ga T | 54.2 ± 1.05 | 52.8 ± 4.60 |
| 4.Lf Gb US | 31.7 ± 0.50 | 20.5 ± 0.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, M.; Şerban, A.; Popa, C.V.; Florescu, M. A Nanoparticle-Based Label-Free Sensor for Screening the Relative Antioxidant Capacity of Hydrosoluble Plant Extracts. Sensors 2019, 19, 590. https://doi.org/10.3390/s19030590
David M, Şerban A, Popa CV, Florescu M. A Nanoparticle-Based Label-Free Sensor for Screening the Relative Antioxidant Capacity of Hydrosoluble Plant Extracts. Sensors. 2019; 19(3):590. https://doi.org/10.3390/s19030590
Chicago/Turabian StyleDavid, Melinda, Adrian Şerban, Claudia V. Popa, and Monica Florescu. 2019. "A Nanoparticle-Based Label-Free Sensor for Screening the Relative Antioxidant Capacity of Hydrosoluble Plant Extracts" Sensors 19, no. 3: 590. https://doi.org/10.3390/s19030590
APA StyleDavid, M., Şerban, A., Popa, C. V., & Florescu, M. (2019). A Nanoparticle-Based Label-Free Sensor for Screening the Relative Antioxidant Capacity of Hydrosoluble Plant Extracts. Sensors, 19(3), 590. https://doi.org/10.3390/s19030590