The Applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile Sensors for Monitoring Different Types of Photopolymerization Processes and Acceleration of Cationic and Free-Radical Photopolymerization Under Near UV Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Absorption and Fluorescence Characteristics
2.3. Electrochemical Characteristics Determination of Oxidation Potentials
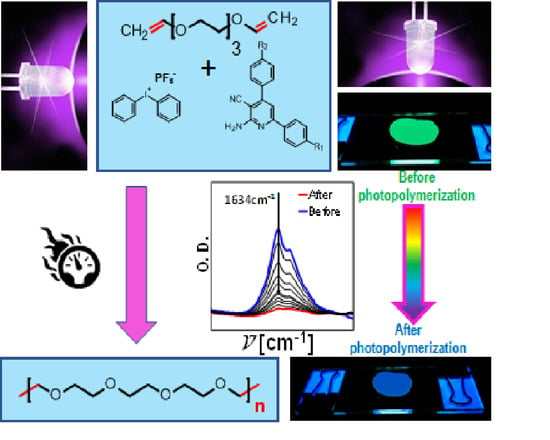
2.4. Preparation of Thin-Layer Samples for Monitoring the Photopolymerization Processes by FPT
2.5. Monitoring the Photopolymerization Processes by FPT
2.6. Monitoring the Photopolymerization Processes by Real-Time FT-IR
2.6.1. Cationic Photopolymerization (CP) Experiments
2.6.2. Free-Radical Photopolymerization (FRP) Experiments
2.6.3. Thiol-ene Photopolymerization (FRP) Experiments
2.6.4. Source of Light for Real-Time Experiments
3. Results
3.1. Spectral Characteristics of the Sensors/Co-Initators
3.2. Performance of the 2-amino-4,6-diphenyl-pyridine-3-carbonitrile derivatives in the Role of Sensors in FPT
3.3. Performance of the 2-amino-4,6-diphenyl-pyridine-3-carbonitrile derivatives in the Role of Co-Initiators in Bimolecular Photoinitiating Systems for Cationic Photopolymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ambrose, W.P.; Goodwin, P.M.; Jett, J.H.; Van Orden, A.; Werner, J.H.; Keller, R.A. Single molecule fluorescence spectroscopy at ambient temperature. Chem. Rev. 1999, 99, 2929–2956. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Plenum Press: New York, NY, USA, 1983; pp. 78–90. [Google Scholar]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2001; pp. 200–348. [Google Scholar]
- Bosch, P.; Catalina, F.; Corrales, T.; Peinado, C. Fluorescent Probes for Sensing Processes in Polymers. Chem. Eur. J. 2005, 11, 4314–4325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamińska, I.; Ortyl, J.; Popielarz, R. Applicability of quinolizino-coumarins for monitoring free radical photopolymerization by fluorescence spectroscopy. Polym. Test. 2015, 42, 99–107. [Google Scholar]
- Ortyl, J.; Sawicz, K.; Popielarz, R. Performance of amidocoumarins as probes for monitoring of cationic photopolymerization of monomers by fluorescence probe technology. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4522–4528. [Google Scholar] [CrossRef]
- Ortyl, J.; Galek, M.; Milart, P.; Popielarz, R. Aminophthalimide probes for monitoring of cationic photopolymerization by fluorescence probe technology and their effect on the polymerization kinetics. Polym. Test. 2012, 31, 466–473. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Brady, T.P.; Lichlyter, D.; Theodorakis, E.A. A Ratiometric Fluorescent Viscosity Sensor. J. Am. Chem. Soc. 2006, 128, 398–399. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-C.; Heo, J.; Woo, H.C.; Lee, J.-A.; Seo, Y.H.; Lee, C.-L.; Kim, S.; Kwon, O.-P. Fluorescent Molecular Rotors for Viscosity Sensors. Chem. Eur. J. 2018, 24, 13706–13718. [Google Scholar]
- Zhou, K.; Ren, M.; Deng, B.; Lin, W. Development of a viscosity sensitive fluorescent probe for real-time monitoring of mitochondria viscosity. New J. Chem. 2017, 41, 11507–11511. [Google Scholar] [CrossRef]
- Lou, J.; Hatton, T.A.; Laibinis, P.E. Fluorescent Probes for Monitoring Temperature in Organic Solvents. Anal. Chem. 1997, 69, 1262–1264. [Google Scholar] [CrossRef]
- Peng, H.; Stich, M.I.J.; Yu, J.; Sun, L.; Fischer, L.H.; Wolfbeis, O.S. Luminescent Europium(III) Nanoparticles for Sensing and Imaging of Temperature in the Physiological Range. Adv. Mater. 2010, 22, 716–719. [Google Scholar] [CrossRef]
- Kozak, M.; Kalota, B.; Tkaczyk, S.; Tsvirko, M. Luminescent Temperature Sensor Material Based on an Eu(III) β-Diketonate Complex Incorporated into Cellulose Triacetate. J. Appl. Spectrosc. 2014, 81, 678–683. [Google Scholar] [CrossRef]
- Lam, H.; Rao, G.; Loureiro, J.; Tolosa, L. Dual Optical Sensor for Oxygen and Temperature Based on the Combination of Time Domain and Frequency Domain Techniques. Talanta 2011, 84, 65–70. [Google Scholar] [CrossRef]
- Baleizao, C.; Nagl, S.; Schaferling, M.; Berberan-Santos, M.N.; Wolfbeis, O.S. Dual Fluorescence Sensor for Trace Oxygen and Temperature with Unmatched Range and Sensitivity. Anal. Chem. 2008, 80, 6449–6457. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, J.; Zhou, L.; Zhou, X.; Xiang, H. Ratiometric optical oxygen sensing: A review in respect of material design. Analyst 2012, 137, 4885–4901. [Google Scholar] [CrossRef] [PubMed]
- Jonaghani, M.Z.; Zali-Boeini, H.; Taheri, R.; Rudbari, H.A.; Askari, B. Naphthothiazole-based highly selective and sensitive fluorescent and colorimetric chemosensor for detection of pollutant metal ions. RSC Adv. 2016, 6, 34940–34945. [Google Scholar] [CrossRef]
- Owicki, J.C. Fluorescence polarization and anisotropy in high throughput screening: Perspectives and primer. J. Biomol. Screen. 2000, 5, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.S.; Jolley, M.E. Fluorescence polarization: An analytical tool for immunoassay and drug discovery. Comb. Chem. High Throughput Screen. 1999, 2, 177–190. [Google Scholar]
- Martinić, I.; Eliseeva, S.V.; Petoud, S. Near-infrared emitting probes for biological imaging: Organic fluorophores, quantum dots, fluorescent proteins, lanthanide(III) complexes and nanomaterials. J. Lumin. 2017, 189, 19–43. [Google Scholar] [CrossRef]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef]
- Ortyl, J.; Galica, M.; Popielarz, R.; Bogdał, D. Application of a carbazole derivative as a spectroscopic fl uorescent probe for real time monitoring of cationic photopolymerization. Pol. J. Chem. Tech. 2014, 16, 75–80. [Google Scholar] [CrossRef]
- Kamińska, I.; Ortyl, J.; Popielarz, R. Mechanism of interaction of coumarin-based fluorescent molecular probes with polymerizing medium during free radical polymerization of a monomer. Polym. Test. 2016, 55, 310–317. [Google Scholar] [CrossRef]
- Kaur, N.; Chopra, S.; Singh, G.; Raj, P.; Bhasin, A.; Sahoo, S.K.; Kuwar, A.; Singh, N. Chemosensors for biogenic amines and biothiols. J. Mater. Chem. B 2018, 6, 4872–4902. [Google Scholar] [CrossRef]
- Gao, Q.; Du, J.; Liu, H.; Lu, S.; Zhou, X.; Yang, C. A hemicyanine-based optical probe for biomembranes and intracellular pH sensing. J. Lumin. 2018, 202, 246–252. [Google Scholar] [CrossRef]
- Khan, R.I.; Pitchumani, K. β-Cyclodextrin included coumarin derivatives as selective fluorescent sensors for Cu2+ ions in HeLa cells. RSC Adv. 2016, 6, 20269–20275. [Google Scholar] [CrossRef]
- Kumar, N.; Bhalla, V.; Kumar, M. Resonance energy transfer-based fluorescent probes for Hg2+, Cu2+ and Fe2+/Fe3+ ions. Analyst 2014, 139, 543–558. [Google Scholar] [CrossRef]
- Szalai, A.M.; Armando, N.G.; Barabas, F.M.; Stefani, F.D.; Giordano, L.; Bari, S.E.; Cavasotto, C.N.; Silberstein, S.; Aramendía, P.F. A fluorescence nanoscopy marker for corticotropin-releasing hormone type 1 receptor: Computer design, synthesis, signaling effects, super-resolved fluorescence imaging, and in situ affinity constant in cells. Phys. Chem. Chem. Phys. 2018, 20, 29212–29220. [Google Scholar] [CrossRef]
- Lin, Q.; Buccella, D. Highly selective, red emitting BODIPY-based fluorescent indicators for intracellular Mg2+ imaging. J. Mater. Chem. B 2018, 6, 7247–7256. [Google Scholar] [CrossRef]
- McLachlan, B.G.; Bell, J.H.; Espina, J.; Gallery, J.; Gouterman, M.; No Demandante, C.G.; Bjarke, L. Flight Testing of a Luminescent Surface Pressure Sensor; NASA Technical Memorandum 103970; Ames Research Center: Mountain View, CA, USA, 1992; pp. 94035–100000.
- Stich, M.I.J.; Fischer, L.H.; Wolfbeis, O.S. Multiple fluorescent chemical sensing and imaging. Chem. Soc. Rev. 2010, 39, 3102–3114. [Google Scholar] [CrossRef]
- Li, M.; Tian, R.; Yan, D.; Liang, R.; Wei, M.; Evans, D.G.; Duan, X. A luminescent ultrathin film with reversible sensing toward pressure. Chem. Commun. 2016, 52, 4663–4666. [Google Scholar] [CrossRef]
- Pączkowski, J. Fluorescent probes as a research tool in polymer chemistry. Polimery 2005, 50, 520–529. [Google Scholar] [CrossRef]
- Józefowicz, M.; Bajorek, A.; Pietrzak, M.; Jędrzejewska, B.; Heldt, J.R.; Heldt, J. Influence of degree of methyl methacrylate polymerization on spectroscopic properties of ethyl 5-(4-aminophenyl)- and 5-(4-dimethylaminophenyl)-3-amino-2,4-dicyanobenzoate. J. Lumin. 2013, 134, 414–422. [Google Scholar] [CrossRef]
- Ortyl, J.; Popielarz, R. The performance of 7-hydroxycoumarin-3-carbonitrile and 7-hydroxycoumarin-3-carboxylic acid as fluorescent probes for monitoring of cationic photopolymerization processes by FPT. J. Appl. Pol. Sci. 2012, 128, 1974–1978. [Google Scholar] [CrossRef]
- Ortyl, J.; Wilamowski, J.; Milart, P.; Galek, M.; Popielarz, R. Relative Sensitization Efficiency of Fluorescent Probes/sensitizers for Monitoring and Acceleration of Cationic Photopolymerization of Monomers. Polym. Test. 2015, 48, 151–159. [Google Scholar] [CrossRef]
- Topa, M.; Ortyl, J.; Chachaj-Brekiesz, A.; Pilch, M.; Popielarz, R. Applicability of samarium(III) complexes for the role of luminescent molecular sensors for monitoring progress of photopolymerization processes and control of the thickness of polymer coatings. Spectrochim. Acta Part A 2018, 199, 30–440. [Google Scholar] [CrossRef]
- Ortyl, J.; Milart, P.; Popielarz, R. Applicability of aminophthalimide probes for monitoring and acceleration of cationic photopolymerization of epoxides. Polym. Test. 2013, 32, 708–715. [Google Scholar] [CrossRef]
- Peinado, C.; Salvador, E.F.; Catalina, F.; Lozano, A.E. Solvatochromic and rigidochromic fluorescent probes based on D–π-A diaryl ethylene and butadiene derivatives for UV-curing monitoring. Polymer 2001, 42, 2815–2825. [Google Scholar] [CrossRef]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers: Part I: Comparison of the performance of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2017, 64, 313–320. [Google Scholar] [CrossRef]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers, Part II: Determination of relative quantum efficiency of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2018, 67, 144–150. [Google Scholar] [CrossRef]
- Itagaki, H.; Horie, K.; Mitra, I. Luminescent probe studies of the microstructure and mobility of solid polymers. Prog. Polym. Sci. 1990, 15, 361–424. [Google Scholar] [CrossRef]
- Wang, Z.J.; Song, J.C.; Bao, R.; Neckers, D.C. Fluorescence probes for monitoring polymerization processes. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 325–333. [Google Scholar] [CrossRef]
- Khudyakov, I.V.; Legg, J.C.; Purvis, M.B.; Overton, B.J. Kinetics of Photopolymerization of Acrylates with Functionality of 1-6. Ind. Eng. Chem. Res. 1999, 38, 3353–3359. [Google Scholar] [CrossRef]
- Hu, S.; Popielarz, R.; Neckers, D.C. Fluorescence Probe Techniques (FPT) for Measuring the Relative Efficiencies of Free-Radical Photoinitiators. Macromolecules 1998, 31, 4107–4113. [Google Scholar] [CrossRef]
- Ortyl, J.; Topa, M.; Kamińska-Borek, I.; Popielarz, R. Mechanism of interaction of aminocoumarins with reaction medium during cationic photopolymerization of triethylene glycol divinyl ether. Eur. Polym. J. 2019, 116, 45–55. [Google Scholar] [CrossRef]
- Lees, A.J. Organometallic complexes as luminescence probes in monitoring thermal and photochemical polymerizations. Coord. Chem. Rev. 1998, 177, 3–35. [Google Scholar] [CrossRef]
- Strehmel, B.; Malpert, J.H.; Sarker, A.M.; Neckers, D.C. New Intramolecular Fluorescence Probes That Monitor Photoinduced Radical and Cationic Cross-Linking. Macromolecules 1999, 32, 7476–7482. [Google Scholar] [CrossRef]













| Structures of the Derivatives of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile | |||||
 |  |  | |||
| S1 | S2 | S3 | |||
 |  |  | |||
| S4 | S5 | S6 | |||
| Reference Molecular Fluorescent Sensors | |||||
 |  | ||||
| C1 | 25ST | ||||
| Compound | Concentration [mol·dm−3] | λab-max [nm] | ε@λab-max [dm3·mol−1·cm−1] | ε@320 nm [dm3·mol−1·cm−1] | λfl @λex-320nm [nm] | Intensity @λfl-max [a. u.] |
|---|---|---|---|---|---|---|
| S1 | 7.38 × 10−5 | 349 | 15,890 | 4835 | 418 | 19,890 |
| S2 | 6.84 × 10−5 | 354 | 12,750 | 6874 | 407 | 15,000 |
| S3 | 7.77 × 10−5 | 357 | 13,780 | 4532 | 409 | 14,580 |
| S4 | 7.37 × 10−5 | 357 | 14,830 | 7068 | 404 | 22,010 |
| S5 | 7.18 × 10−5 | 364 | 13,990 | 4668 | 448 | 9610 |
| S6 | 6.99 × 10−5 | 363 | 12,460 | 6473 | 432 | 27,420 |
| Sensor | λmax-BEFORE [nm] | Intensity @λmax-BEFORE [a.u.] | λmax-AFTER POL [nm] | Intensity @λmax-AFTER [a.u.] | |ΔImax| [a.u.] | ΔImax a [%] | Δλmax [nm] | Relative Sensitivity b |
| Free-radical photopolymerization process of TMPTMA under 320 nm | ||||||||
| S1 | 413 | 35,770 | 404 | 29,800 | 5970 | 17 | 9 | 2.15 |
| S2 | 407 | 36,530 | 401 | 27,220 | 9310 | 25 | 6 | 1.56 |
| S3 | 413 | 63,550 | 405 | 54,390 | 9160 | 14 | 8 | 1.55 |
| S4 | 406 | 48,980 | 402 | 37,910 | 11,070 | 23 | 4 | 1.32 |
| S5 | 440 | 16,630 | 427 | 15,180 | 1450 | 9 | 13 | 2.76 |
| S6 | 426 | 39,030 | 420 | 28,200 | 10,830 | 28 | 6 | 1.77 |
| C1-ref. | 423 | 22,900 | 418 | 12,920 | 9980 | 44 | 5 | 1.00 |
| Sensor | λmax-BEFORE [nm] | Intensity λmax-BEFORE [a.u.] | λmax-AFTER [nm] | Intensity @λmax-AFTER [a.u.] | |ΔImax| [a.u.] | ΔImax a [%] | Δλmax [nm] | Relative Sensitivity b |
| Thiol-ene photopolymerization process of TMPTMA/MERCAPTO under 320 nm | ||||||||
| S1 | 413 | 21,950 | 405 | 24,290 | 2340 | 11 | 8 | 0.53 |
| S2 | 409 | 38,060 | 403 | 42,850 | 4790 | 13 | 6 | 1.39 |
| S3 | 414 | 33,000 | 406 | 39,340 | 6340 | 19 | 8 | 1.49 |
| S4 | 407 | 56,550 | 403 | 65,970 | 9420 | 17 | 4 | 1.12 |
| S5 | 442 | 19,030 | 428 | 25,280 | 6250 | 33 | 14 | 2.93 |
| S6 | 428 | 50,860 | 423 | 51,330 | 470 | 1 | 5 | 1.44 |
| C1-ref. | 426 | 26,910 | 421 | 41,190 | 14,280 | 53 | 5 | 1.00 |
| Sensor | λmax-BEFORE [nm] | Intensity λmax-BEFORE [a.u.] | λmax-AFTER [nm] | Intensity @λmax-AFTER [a.u.] | |ΔImax| [a.u.] | ΔImax a [%] | Δλmax [nm] | |
| Cationic photopolymerization process of TEGDVE under 320 nm | ||||||||
| S1 | 420 | 11,760 | 423 | 40,050 | 28,290 | 241 | 3 | |
| S2 | 415 | 12,580 | 426 | 17,210 | 4630 | 37 | 11 | |
| S3 | 417 | 12,220 | 447 | 38,940 | 26,720 | 219 | 30 | |
| S4 | 412 | 16,110 | 442 | 26,270 | 10,160 | 63 | 30 | |
| S5 | 450 | 16,540 | 463 | 54,140 | 37,600 | 227 | 13 | |
| S6 | 438 | 11,910 | 441 | 23,090 | 11,180 | 94 | 3 | |
| 25ST-ref. | 459 | 11,180 | 440 | 9790 | 1390 | 12 | 19 | |
| Fluorescent Sensor/ Co-initiator | ε @λab-365 [dm3·mol−1·cm−1] | Eox1/2 [mV] | E00 [eV] | ΔGet [eV] | Conversion of Double Bonds in TEGDVE @365 nm (95 mW/cm2) | Conversion of CADE Epoxy Monomer @365 nm (190 mW/cm2) |
|---|---|---|---|---|---|---|
| S1 | 8410 | 1584 | 3.22 | −0.92 | 83 | 50 |
| S2 | 10,330 | 1635 | 3.10 | −0.74 | 83 | 71 |
| S3 | 12,970 | 1521 | 3.06 | −0.82 | 82 | 78 |
| S4 | 13,820 | 1602 | 3.09 | −0.77 | 83 | 71 |
| S5 | 13,990 | 1157 | 3.06 | −1.18 | 85 | 78 |
| S6 | 12,410 | 1664 | 3.10 | −0.71 | 82 | 72 |
| Fluorescent Sensor/ Co-Initiator | Free-Radical Photopolymerization | Thiol-ene Photopolymerization | |
|---|---|---|---|
| Conversion of Double Bonds in TMPTA @365 nm (95 mW/cm2) | Conversion of Double Bonds in TMPTMA in thiol-ene Polymerization @365 nm (95 mW/cm2) | Conversion of thiol Bonds in Mercapto in thiol-ene Polymerization @365 nm (95 mW/cm2) | |
| S1 | 44 | 93 | 45 |
| S2 | 46 | 73 | 35 |
| S3 | 25 | 93 | 46 |
| S4 | 49 | 92 | 45 |
| S5 | 30 | 95 | 45 |
| S6 | 47 | 89 | 44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortyl, J.; Fiedor, P.; Chachaj-Brekiesz, A.; Pilch, M.; Hola, E.; Galek, M. The Applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile Sensors for Monitoring Different Types of Photopolymerization Processes and Acceleration of Cationic and Free-Radical Photopolymerization Under Near UV Light. Sensors 2019, 19, 1668. https://doi.org/10.3390/s19071668
Ortyl J, Fiedor P, Chachaj-Brekiesz A, Pilch M, Hola E, Galek M. The Applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile Sensors for Monitoring Different Types of Photopolymerization Processes and Acceleration of Cationic and Free-Radical Photopolymerization Under Near UV Light. Sensors. 2019; 19(7):1668. https://doi.org/10.3390/s19071668
Chicago/Turabian StyleOrtyl, Joanna, Paweł Fiedor, Anna Chachaj-Brekiesz, Maciej Pilch, Emilia Hola, and Mariusz Galek. 2019. "The Applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile Sensors for Monitoring Different Types of Photopolymerization Processes and Acceleration of Cationic and Free-Radical Photopolymerization Under Near UV Light" Sensors 19, no. 7: 1668. https://doi.org/10.3390/s19071668
APA StyleOrtyl, J., Fiedor, P., Chachaj-Brekiesz, A., Pilch, M., Hola, E., & Galek, M. (2019). The Applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile Sensors for Monitoring Different Types of Photopolymerization Processes and Acceleration of Cationic and Free-Radical Photopolymerization Under Near UV Light. Sensors, 19(7), 1668. https://doi.org/10.3390/s19071668