A Point-of-Care Based on Label-Free Interferometric Optical Detection Method to Evaluate Interferon Gamma (IFN-γ): A Correlation with the ELISA Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. BICELLs Fabrication and Materials
2.2. Point-Of-Car: Optical Read-Out Device
2.3. Assay Protocol: ELISA Technique and Point-Of-Care (IROP)
2.3.1. ELISA Technique for Interferon Gamma detection
2.3.2. Point of Care Protocol for Interferon Gamma Detection
3. Results and Discussion
3.1. Optimization of Sensing Surface
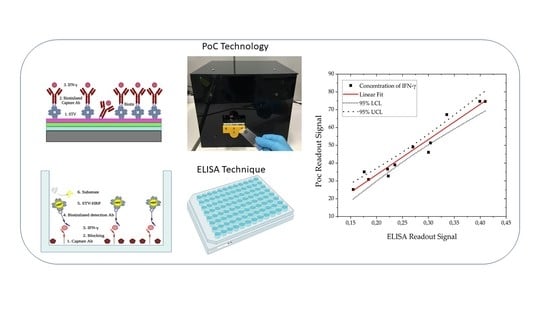
3.2. Detection of Interferon-Gamma. Comparison of ELISA and PoC Techniques
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctot, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Biron, C.A.; Nguyen, K.B.; Pien, G.C.; Cousens, L.P.; Salazar-Mather, T.P. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annu. Rev. Immunol. 1999, 17, 189–220. [Google Scholar] [CrossRef]
- Omae, T.; Saito, Y.; Tsuchie, H.; Ohno, K.; Maegaki, Y.; Sakuma, H. Cyokine/chemokine elevation during the transition phase from HSV encephalitis to autoimmune anti-NMDA receptor encephalitis. Brain Dev. 2018, 40, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Jager, W.; Velthius, H.; Prakken, B.J.; Kuis, W.; Rijkers, G.T. Simultaneous Detection of 15 Human Cytokines in a Single Sample of Stimulated Peripheral Blood Mononuclear Cells. Clin. Diagn. Lab. Immunol. 2003, 10, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Cohen, L.; Wang, J.; Walt, D.R. Competitive Immunoassays for the Detection of Small Molecules Using Single Molecule Arrays. J. Am. Chem. Soc. 2018, 140, 18132–18139. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.; Lindenmann, J. Virus interference. I. The interferon. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1957, 147, 258–267. [Google Scholar] [CrossRef]
- Pestka, S.; Krause, C.; Walter, M. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2005, 202, 8–32. [Google Scholar] [CrossRef]
- Dorman, S.E.; Holland, S.M. Interferon-g and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 2000, 11, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997, 15, 749–795. [Google Scholar] [CrossRef]
- Sen, G.C. Viruses and Interferons. Annu. Rev. Microbiol. 2001, 55, 255–281. [Google Scholar] [CrossRef]
- Lavalvani, A.; Pareek, M. Interferon gamma release assays: Principles and practice. Enferm. Infecc. Microbiol. Clín. 2010, 28, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Pandya, Y.; Moolchan, K.; Lavoie, T.B. Interferon Gamma: Activity and Elisa Detection Comparisons. Biotechniques 2008, 45, 101–102. [Google Scholar] [CrossRef]
- Favre, N.; Bordmann, G.; Rudin, W. Comparison of cytokine measurements using ELISA, ELISPOT and SemiQuantitative RT-PCR. J. Immunol. Methods 1997, 204, 57–66. [Google Scholar] [CrossRef]
- Tuleuova, N.; Jones, C.N.; Yan, J.; Ramanculov, E.; Yokobayashi, Y.; Revzin, A. Development of an Aptamer Beacon for Detection of Interferon-Gamma. Anal. Chem. 2010, 82, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Farid, S.; Meshik, X.; Choi, M.; Mukherjee, S.; Lan, Y.; Parikh, D.; Poduri, S.; Baterdene, U.; Huang, C.E.; Wang, Y.Y.; et al. Detection of Interferon Gamma Using Graphene an Aptamer based FET-like Electrochemical Biosensor. Biosens. Bioelectron. 2015, 71, 294–299. [Google Scholar] [CrossRef]
- Ding, S.; Cargill, A.; Das, S.; Medintz, I.; Claussen, J. Biosensing with Förster Resonance Energy Transfer Coupling between fluorophores and Nanocarbon allotropes. Sensors 2015, 15, 14766–14787. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Pui, T.S.; Kongsuphol, P.; Tang, K.C.; Arya, S.K. Aptamer-Based Array Electrodes for Quantitative Interferon-gamma Detection. Biosens. Bioelectron. 2014, 53, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jian, Z.; Chenm, X.; Li, J.; Qin, P.; Zhao, J.; Jiao, X.; Hu, X. Electrochemical Impedance Immunosensor for Sub-Picogram Level Detection of Bovine Interferon Gamma Based on Cylinder-Shaped TiO2 Nanorods. Biosens. Bioelectron. 2015, 63, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Mosher, C.; Lee Xian, L.; Das Suprem, R.; Cargill Allison, A.; Tang, X.; Chen, B.; McLamore Eric, S.; Gomes, C.; Hostetter Jesse, M.; et al. Rapid and Label-Free Detection of Interferon Gamma via an Electrochemical Aptasensor Comprising a Ternary Surface Monolayer on a Gold Interdigitated Electrode Array. ACS Sens. 2017, 2, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Lei, S.; Liu, Z.; Ouyang, G.; Zou, L.; Ye, B. A label-free IFN-γ aptasensor based on target-triggered allosteric switching of aptamer beacon and streptavidin-inorganic hybrid composites. Anal. Chim. Acta 2019, 1087, 29–35. [Google Scholar] [CrossRef]
- Liu, M.W.; Chang, H.J.; Lee, S.S.; Lee, C.K. Cross-calibrating Interferon-gamma detection by using electrochemical impedance spectroscopy and paraboloidal mirror enabled surface plasmon resonance interferometer. In Proceedings of the SPIE 9701, 97010U, San Francisco, CA, USA, 10 March 2016. [Google Scholar] [CrossRef]
- Singh, M.; Troung, J.; Reeves, W.B.; Hahm, J.I. Emerging Cytokine Biosnsors with Optical Detection Modalities and Nanomaterial-Enabled Signal Enhancement. Sensors 2017, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-H.; Erdene, N.; Park, J.-H.; Jeong, D.H.; Lee, H.-Y.; Lee, S.K. Real-time label-free immunoassay of interferon-gamma and prostate-specific antigen using a Fiber-Optic Localized Surface Plasmon Resonance sensor. Biosens. Bioelectron. 2012, 39, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Song, C.; Que, L. Label-free monitoring of Alzheimer´s disease biomarkers in cerebrospinal fluid. In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology (IEEE-NANO), Pittsburgh, PA, USA, 25–28 July 2017; pp. 1074–1077. [Google Scholar]
- Barozzi, M.; Manicardi, A.; Vannucci, A.; Candiani, A.; Sozzi, M.; Konstantaki, M.; Pissadakis, S.; Corradini, R.; Selleri, S.; Cucinotta, A. Optical fiber sensors for Label-free DNA Detection. J. Lightwave Technol. 2016, 35, 3461–3472. [Google Scholar] [CrossRef]
- Lei, C.; Guo, B.; Jiang, Y.; Wu, Y.; Kobayashi, H.; Ito, T.; Yasumoto, A.; Yatomi, Y.; Ozeki, Y.; Goda, K. High-throughput, label-free, multivariate cell analysis with optofluidic time-strech microscopy. In Proceedings of the 2017 Conference on Lasers and Electro-Optics Pacific Rim (CLEO-PR), Singapore, 31 July–4 August 2017. [Google Scholar] [CrossRef]
- Holgado, M.; Sanza, F.J.; Hernandez, A.L.; Lavín, A.; Casquel, R.; Laguna, M.F. Description of an advantageous optical label-free biosensing interferometric read-out method to measure biological species. Sensors 2014, 14, 3675–3689. [Google Scholar] [CrossRef]
- Holgado, M.; Maigler, M.V.; Santamaría, B.; Hernandez, A.L.; Lavín, A.; Laguna, M.F.; Sanza, F.J.; Granados, D.; Casquel, R.; Portilla, J.; et al. Towards reliable optical label-free point-of-care (PoC) biosensing devices. Sens. Actuators B Chem. 2016, 236, 765–772. [Google Scholar] [CrossRef]
- Casquel, R.; Holgado, M.; Laguna, M.F.; Hernández, A.L.; Santamaría, B.; Lavín, A.; Tramarín, L.; Herreros, P. Engineering vertically interrogated interferometric sensors for optical label-free biosensing. Anal. Bioanal. Chem. 2020, 412, 3285–3297. [Google Scholar] [CrossRef] [Green Version]
- Maigler, M.V.; Holgado, M.; Laguna, M.F.; Sanza, F.J.; Santamaría, B.; Lavín, A.; Espinosa, R.L. A new device based on Interferometric Optical Detection Method for Label-Free Screening of C-Reactive Protein. IEEE Trans. Instrum. Meas. 2019, 68, 3193–3199. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, R.L.; Laguna, M.F.; Fernandez, F.; Santamaría, B.; Sanza, F.J.; Maigler, M.V.; Alvarez-Millan, J.J.; Canalejas-Tejero, V.; Holgado, M. A Proof-of-Concept of Label-Free Biosensing System for Food Allergy Diagnostics in Biophotonic Sensing Cells: Performance Comparison with ImmunoCAP. Sensors 2018, 18, 2686. [Google Scholar] [CrossRef] [Green Version]
- Lavin, A.; Casquel, R.; Sanza, F.J.; Laguna, M.F.; Holgado, M. Efficient design and optimization of bio-photonic sensing cells (BICELLs) for label free biosensing. Sens. Actuators B Chem. 2013, 176, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Santamaría, B.; Laguna, M.F.; López-Romero, D.; Hernandez, A.L.; Sanza, F.J.; Lavín, A.; Casquel, R.; Maigler, M.V.; Espinosa, R.L.; Holgado, M. Development towards Compact Nitrocellulose-Based Interferometric Biochips for Dry Eye MMP9 Label-Free In-Situ Diagnosis. Sensors 2017, 17, 1158–1164. [Google Scholar] [CrossRef]
- Blankenburg, R.; Meller, P.; Ringsdorf, H.; Salesse, C. Interaction between biotin lipids and streptavidin in monolayers: Formation of oriented two-dimensional protein domains induced by surface recognition. Biochemistry 1989, 28, 8214–8221. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.C.; Ohlendorf, D.H.; Wendoloski, J.J.; Salemme, F.R. Structural origins of high-affinity biotin binding of streptavidin. Science 1989, 243, 85–88. [Google Scholar] [CrossRef] [PubMed]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heras, M.F.L.; Ramirez, Y.; Fernández Martín, C.; L. Espinosa, R.; Lavín, A.; Holgado, M. A Point-of-Care Based on Label-Free Interferometric Optical Detection Method to Evaluate Interferon Gamma (IFN-γ): A Correlation with the ELISA Technique. Sensors 2020, 20, 4776. https://doi.org/10.3390/s20174776
Heras MFL, Ramirez Y, Fernández Martín C, L. Espinosa R, Lavín A, Holgado M. A Point-of-Care Based on Label-Free Interferometric Optical Detection Method to Evaluate Interferon Gamma (IFN-γ): A Correlation with the ELISA Technique. Sensors. 2020; 20(17):4776. https://doi.org/10.3390/s20174776
Chicago/Turabian StyleHeras, María Fe Laguna, Yolanda Ramirez, Celia Fernández Martín, Rocío L. Espinosa, Alvaro Lavín, and Miguel Holgado. 2020. "A Point-of-Care Based on Label-Free Interferometric Optical Detection Method to Evaluate Interferon Gamma (IFN-γ): A Correlation with the ELISA Technique" Sensors 20, no. 17: 4776. https://doi.org/10.3390/s20174776
APA StyleHeras, M. F. L., Ramirez, Y., Fernández Martín, C., L. Espinosa, R., Lavín, A., & Holgado, M. (2020). A Point-of-Care Based on Label-Free Interferometric Optical Detection Method to Evaluate Interferon Gamma (IFN-γ): A Correlation with the ELISA Technique. Sensors, 20(17), 4776. https://doi.org/10.3390/s20174776