A Comparative Study on the Oxidation of Label-Free Silver Triangular Nanoplates by Peroxides: Main Effects and Sensing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Synthesis of Silver Triangular Nanoplates in Aqueous Solution
2.3. Procedures
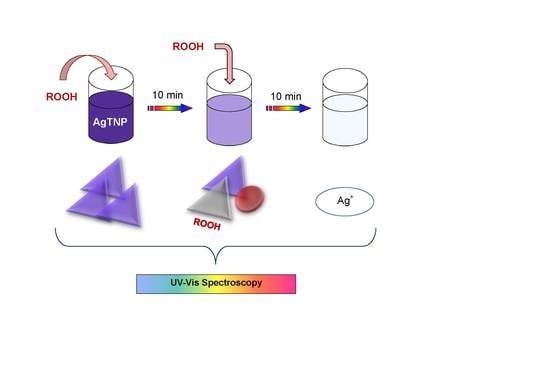
3. Results and Discussion
3.1. Interaction of Peroxides with Label-Free Silver Triangular Nanoplates
3.1.1. Effect of pH
3.1.2. Effect of the Interaction Time
3.1.3. Effect of AgTNPs Concentration
3.2. Spectrophotometric Determination of Peroxides
3.2.1. Analytical Performance
3.2.2. Selectivity Studies
3.2.3. Sample Analysis
3.2.4. Comparison with Other Methods for the Determination of Peroxides
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pillay, C.S.; Eagling, B.D.; Driscoll, S.R.; Rohwer, J.M. Quantitative measures for redox signaling. Free. Rad. Biol. Med. 2016, 96, 290–303. [Google Scholar] [CrossRef]
- Briehl, M.M. Oxygen in human health from life to death—An approach to teaching redox biology and signaling to graduate and medical students. Redox. Biol. 2015, 5, 124–139. [Google Scholar] [CrossRef] [Green Version]
- Olenin, A.Y.; Olenina, E.G. Spectrophotometric nonenzymatic determination of hydrogen peroxide using silver nanoparticles. J. Anal. Chem. 2017, 72, 234–238. [Google Scholar] [CrossRef]
- Muráth, S.; Alsharif, N.B.; Sáringer, S.; Katana, B.; Somosi, Z.; Szilagyi, I. Antioxidant materials based on 2D nanostructures: A review on recent progresses. Crystals 2020, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Beloborodova, N.; Bairamov, I.; Olenin, A.; Shubina, V.; Teplova, V.; Fedotcheva, N. Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J. Biomed. Sci. 2012, 19, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chang, T.S.; Jeong, W.; Kang, D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells 2010, 29, 539–549. [Google Scholar] [CrossRef]
- Guo, H.; Aleyasin, H.; Dickinson, B.C.; Haskew-Layton, R.E.; Ratan, R.R. Recent advances in hydrogen peroxide imaging for biological applications. Cell. Biosci. 2014, 4, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Apyari, V.V.; Dmitrienko, S.G.; Gorbunova, M.V.; Furletov, A.A.; Zolotov, Y.A. Gold and silver nanoparticles in optical molecular absorption spectroscopy. J. Anal. Chem. 2019, 74, 21–32. [Google Scholar] [CrossRef]
- Cialla, D.; März, A.; Böhme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay. A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.C.; Yu, A.B. Silver nanoplates: A highly sensitive material toward inorganic anions. Langmuir 2008, 24, 4300–4309. [Google Scholar] [CrossRef] [PubMed]
- Rubira, R.J.G.; Camacho, S.A.; Martin, C.S.; Mejía-Salazar, J.R.; Reyes Gómez, F.; da Silva, R.R.; Oliveira Junior, O.N.; Alessio, P.; Constantino, C.J.L. Designing silver nanoparticles for detecting levodopa (3,4-dihydroxyphenylalanine, L-dopa) using surface-enhanced Raman scattering (SERS). Sensors 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiva Prasad, K.; Shruthi, G.; Shivamallu, C. Functionalized silver nano-sensor for colorimetric detection of Hg2+ ions: Facile synthesis and docking studies. Sensors 2018, 18, 2698. [Google Scholar] [CrossRef] [Green Version]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar] [CrossRef]
- Wang, C.; Bi, X.; Wang, M.; Zhao, X.; Lin, Y. Dual-channel online optical detection platform integrated with a visible light absorption approach for continuous and simultaneous in vivo monitoring of ascorbic acid and copper(II) ions in a living rat brain. Anal. Chem. 2019, 91, 16010–16016. [Google Scholar] [CrossRef]
- Yan, P.; Ding, Z.; Li, X.; Dong, Y.; Fu, T.; Wu, Y. Colorimetric sensor array based on Wulff-type boronate functionalized AgNPs at various pH for bacteria identification. Anal. Chem. 2019, 91, 12134–12137. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Chen, P.; Chen, S.; Yuan, Z.; Yu, C.; Ren, B.; Zhang, K. In situ study of the antibacterial activity and mechanism of action of silver nanoparticles by surface-enhanced Raman spectroscopy. Anal. Chem. 2013, 85, 5436–5443. [Google Scholar] [CrossRef]
- Markina, N.E.; Markin, A.V.; Zakharevich, A.M.; Gorin, D.A.; Rusanova, T.Y.; Goryacheva, I.Y. Multifunctional silver nanoparticle-doped silica for solid-phase extraction and surface-enhanced Raman scattering detection. J. Nanopart. Res. 2016, 18, 353–362. [Google Scholar] [CrossRef]
- Komova, N.S.; Pavlov, A.M.; Serdobintsev, A.A.; Galushka, V.V.; Rusanova, T.Y. SERS-platforms based on electrospun nanofibers with embedded silver nanoparticles. Proc. SPIE 2019, 11067, 110671A-1–110671A-7. [Google Scholar] [CrossRef]
- Teerasong, S.; Sani, M.; Numsawat, P.; Martchoo, R.; Chompoosor, A.; Nacapricha, D. A silver nanoparticle thin film modified glass substrate as a colourimetric sensor for hydrogen peroxide. J. Exp. Nanosci. 2015, 10, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Teerasong, S.; Sonsa-Ard, T.; Vimolkanjana, C.; Choengchan, N.; Chompoosor, A.; Nacapricha, D. Colorimetric sensor using silver nanoparticles for determination of hydrogen peroxide based on a flow injection system. J. Nanoelectron. Optoelectron. 2013, 8, 446–449. [Google Scholar] [CrossRef]
- Apyari, V.V.; Terenteva, E.A.; Kolomnikova, A.R.; Garshev, A.V.; Dmitrienko, S.G.; Zolotov, Y.A. Potentialities of differently-stabilized silver nanoparticles for spectrophotometric determination of peroxides. Talanta 2019, 202, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Terenteva, E.A.; Apyari, V.V.; Dmitrienko, S.G.; Garshev, A.V.; Volkov, P.A.; Zolotov, Y.A. Determination of pyrophosphate and sulfate using polyhexamethylene guanidine hydrochloride-stabilized silver nanoparticles. Talanta 2018, 180, 346–351. [Google Scholar] [CrossRef]
- Shen, J.; Sun, C.; Wu, X. Silver nanoprisms-based Tb(III) fluorescence sensor for highly selective detection of dopamine. Talanta 2017, 165, 369–376. [Google Scholar] [CrossRef]
- Apyari, V.V.; Gorbunova, M.O.; Shevchenko, A.V.; Furletov, A.A.; Volkov, P.A.; Garshev, A.V.; Dmitrienko, S.G.; Zolotov, Y.A. Towards highly selective detection using metal nanoparticles: A case of silver triangular nanoplates and chlorine. Talanta 2018, 176, 406–411. [Google Scholar] [CrossRef]
- Gorbunova, M.O.; Baulina, A.A.; Kulyaginova, M.S.; Apyari, V.V.; Furletov, A.A.; Volkov, P.A.; Bochenkov, V.E.; Starukhin, A.S.; Dmitrienko, S.G. Dynamic gas extraction of iodine in combination with a silver triangular nanoplate-modified paper strip for colorimetric determination of iodine and of iodine-interacting compounds. Microchim. Acta 2019, 186, 188–197. [Google Scholar] [CrossRef]
- Furletov, A.A.; Apyari, V.V.; Garshev, A.V.; Volkov, P.A.; Tolmacheva, V.V.; Dmitrienko, S.G. Sorption of triangular silver nanoplates on polyurethane foam. Russ. J. Phys. Chem. 2018, 92, 357–360. [Google Scholar] [CrossRef]
- Feng, H.; Dong, J.; Wu, X.; Yang, F.; Ma, L.; Liu, X.; Liu, Q. Ultra-large local field enhancement effect of isolated thick triangular silver nanoplates on a silicon substrate in the green waveband. Opt. Lett. 2020, 45, 2099–2102. [Google Scholar] [CrossRef]
- Chen, L.; Fu, X.; Lu, W.; Chen, L. Highly sensitive and selective colorimetric sensing of Hg2+ based on the morphology transition of silver nanoprisms. ACS Appl. Mater. Interfaces 2013, 5, 284–290. [Google Scholar] [CrossRef]
- Xue, B.; Wang, D.; Zuo, J.; Kong, X.; Zhang, Y.; Liu, X.; Tu, L.; Chang, Y.; Li, C.; Wu, F.; et al. Towards high quality triangular silver nanoprisms: Improved synthesis, six-tip based hot spots and ultra-high local surface plasmon resonance sensitivity. Nanoscale 2015, 7, 8048–8057. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, H. A novel triangular silver nanoprisms-based surface plasmon resonance assay for free chlorine. Analyst 2015, 140, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Brandon, M.P.; Ledwith, D.M.; Kelly, J.M. Preparation of saline-stable, silica-coated triangular silver nanoplates of use for optical sensing. J. Colloid. Interface. Sci. 2014, 415, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, C.; Wu, Q.; Li, K.; Tan, L. Application of triangular silver nanoplates for colorimetric detection of H2O2. Sens. Actuat. B Chem. 2015, 220, 314–317. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L. Colorimetric detection of hydrogen peroxide using silver nanoparticles with three different morphologies. Anal. Methods 2016, 8, 6691–6695. [Google Scholar] [CrossRef]
- Métraux, G.S.; Mirkin, C.A. Rapid thermal synthesis of silver nanoprisms with chemically tailorable thickness. Adv. Mater. 2005, 17, 412–415. [Google Scholar] [CrossRef]
- Mitsai, E.; Kuchmizhak, A.; Pustovalov, E.; Sergeev, A.; Mironenko, A.; Bratskaya, S.; Linklater, D.P.; Balcytis, A.; Ivanova, E.; Juodkazis, S. Chemically non-perturbing SERS detection of a catalytic reaction with black silicon. Nanoscale 2018, 10, 9780–9787. [Google Scholar] [CrossRef] [Green Version]
- Millstone, J.E.; Hurst, S.J.; Metraux, G.S.; Cutler, J.I.; Mirkin, C.A. Colloidal gold and silver triangular nanoprisms. Small 2009, 5, 646–664. [Google Scholar] [CrossRef]
- Sanz, V.; de Marcos, S.; Galbán, J. Hydrogen peroxide and peracetic acid determination in waste water using a reversible reagentless biosensor. Anal. Chim. Acta 2007, 583, 332–339. [Google Scholar] [CrossRef]
- Lim, D.S.; Lee, Y.J.; Yoo, J.H.; Choi, M.G.; Paek, K.; Koh, H.R.; Chang, S.-K. Fluorometric analysis of peracetic acid by the oxidative hydroxylation of a phenylboronic acid containing dye. Sens. Actuat. B Chem. 2019, 298, 126824. [Google Scholar] [CrossRef]
- Roy, K.; Sarkar, C.K.; Ghosh, C.K. Fast colourimetric detection of H2O2 by biogenic silver nanoparticles synthesized using Benincasa hispida fruit extract. Nanotechnol. Rev. 2016, 5, 251–258. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Hieda, K.; Ikeda, K.; Tamiya, E. Hydrogen peroxide detection with a silver nanoparticle grating chip fabricated by plasmonic plating. Anal. Methods 2019, 11, 2991–2996. [Google Scholar] [CrossRef]
- Gatselou, V.A.; Giokas, D.L.; Vlessidis, A.G.; Prodromidis, M.I. Rhodium nanoparticle-modified screen-printed graphite electrodes for the determination of hydrogen peroxide in tea extracts in the presence of oxygen. Talanta 2015, 134, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Wang, B.D.; Yin, J.; Chen, X.F.; Tang, Y.G. Electrochemical tracking hydrogen peroxide secretion in live cells based on autocatalytic oxidation reaction of silver nanoparticles. Electrochem. Commun. 2015, 53, 37–40. [Google Scholar] [CrossRef]






| Chemical Name | MW, g mol−1 | Structure | Type a |
|---|---|---|---|
| Hydrogen peroxide (HP) | 34.01 |  | Unsubstituted |
| tert-Butyl hydroperoxide (TBHP) | 90.12 |  | Monosubstituted, EDS |
| Peracetic acid (PAA) | 76.05 |  | Monosubstituted, EAS |
| di-tert-Butyl peroxide (DTBP) | 146.23 |  | Disubstituted, EDS/EDS |
| tert-Butyl peroxybenzoate (TBPB) | 194.23 |  | Disubstituted, EDS/EAS |
| Analyte | k, L mmol−1 | r2 | LOD, μmol L−1 | Determination Range, μmol L−1 | RSD a, % | RSD b, % |
|---|---|---|---|---|---|---|
| PAA | 106 | 0.990 | 0.08 | 0.25–6 | 3 | 11.3 |
| HP | 5.70 | 0.991 | 1.6 | 5–60 | 2 | 10.5 |
| TBHP | 0.376 | 0.990 | 24 | 72–600 | 3 | 11.1 |
| Sample | Content of Hydrogen Peroxide, wt.% | t-test Value c | ||||
|---|---|---|---|---|---|---|
| Declared by Manufacturer | AgTNPs-Based Method | Alternative Method | ||||
| Found | RSD% | Found | RSD% | |||
| Hydroperite formulation a | 36.2 | 36 ± 4 | 5 | 36 ± 2 | 2 | 0 |
| Estel De Luxe hair oxygen b | 9.0 | 8.8 ± 0.9 | 4 | 9.3 ± 0.6 | 2 | 1.98 |
| Sample | Content of tert-Butyl Hydroperoxide, mg g−1 | RSD% | |
|---|---|---|---|
| Added | Found | ||
| Model mixture based on tert-butanol spiked with TBHP | 0 | 0 | — |
| 1.2 | 1.1 ± 0.2 | 8 | |
| Analyte | Method | LOD, μmol L−1 | Determination Range, μmol L−1 | Reference |
|---|---|---|---|---|
| PAA | Spectrophotometry | 0.6 | 0.6–100 | [39] |
| Fluorimetry | 0.04 | 0.1–20 | [40] | |
| Present method | 0.08 | 0.25–6 | This article | |
| HP | Spectrophotometry | 1000 | 1000–100,000 | [21] |
| Visual colorimetry | 1000 | 1000–10,000 | [41] | |
| Spectrophotometry | 80 | 500–15,000 | [3] | |
| Spectrophotometry | 18 | 29–150; 290–590 | [22] | |
| Light diffraction | 8 | 10–670 | [42] | |
| Voltammetry | 2 | 5–600 | [43] | |
| Sweep voltammetry | 0.5 | 1–300 | [44] | |
| Spectrophotometry | 0.00136 | 0.05–1 | [34] | |
| Spectrophotometry | 0.00037 | 10–500 | [35] | |
| Present method | 1.6 | 5–60 | This article |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furletov, A.; Apyari, V.; Garshev, A.; Dmitrienko, S. A Comparative Study on the Oxidation of Label-Free Silver Triangular Nanoplates by Peroxides: Main Effects and Sensing Applications. Sensors 2020, 20, 4832. https://doi.org/10.3390/s20174832
Furletov A, Apyari V, Garshev A, Dmitrienko S. A Comparative Study on the Oxidation of Label-Free Silver Triangular Nanoplates by Peroxides: Main Effects and Sensing Applications. Sensors. 2020; 20(17):4832. https://doi.org/10.3390/s20174832
Chicago/Turabian StyleFurletov, Aleksei, Vladimir Apyari, Alexey Garshev, and Stanislava Dmitrienko. 2020. "A Comparative Study on the Oxidation of Label-Free Silver Triangular Nanoplates by Peroxides: Main Effects and Sensing Applications" Sensors 20, no. 17: 4832. https://doi.org/10.3390/s20174832
APA StyleFurletov, A., Apyari, V., Garshev, A., & Dmitrienko, S. (2020). A Comparative Study on the Oxidation of Label-Free Silver Triangular Nanoplates by Peroxides: Main Effects and Sensing Applications. Sensors, 20(17), 4832. https://doi.org/10.3390/s20174832