Vanadyl Phthalocyanine Films and Their Hybrid Structures with Pd Nanoparticles: Structure and Sensing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phthalocyanine Films Preparation
2.2. Deposition of Pd Nanoparticles
2.3. Characterization of Thin Films
2.4. Study of Sensor Properties
3. Results and Discussion
3.1. Structure of Vanadyl Phthalocyanines (VOPc and VOPcF4) Thin Films
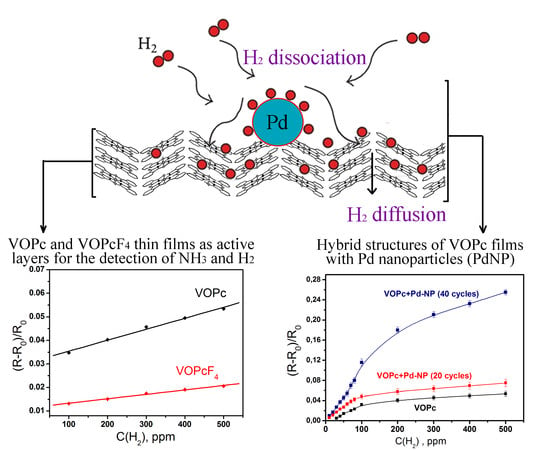
3.2. Sensor Response of VOPc and VOPcF4 Films to Ammonia and Hydrogen
3.3. Hybrid Structures of VOPc with Pd Nanoparticles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dong, Z.; Kong, X.; Wu, Y.; Zhang, J.; Chen, Y. High-Sensitive Room-Temperature NO2 Sensor Based on a Soluble n-Type Phthalocyanine Semiconductor. Inorg. Chem. Commun. 2017, 77, 18–22. [Google Scholar] [CrossRef]
- Kabwe, K.P.; Louzada, M.; Britton, J.; Olomola, T.O.; Nyokong, T.; Khene, S. Nonlinear Optical Properties of Metal Free and Nickel Binuclear Phthalocyanines. Dye. Pigment. 2019, 168, 347–356. [Google Scholar] [CrossRef]
- Suzuki, A.; Okumura, H.; Yamasaki, Y.; Oku, T. Fabrication and Characterization of Perovskite Type Solar Cells Using Phthalocyanine Complexes. Appl. Surf. Sci. 2019, 488, 586–592. [Google Scholar] [CrossRef]
- Kumar, A.; Brunet, J.; Varenne, C.; Ndiaye, A.; Pauly, A. Phthalocyanines Based QCM Sensors for Aromatic Hydrocarbons Monitoring: Role of Metal Atoms and Substituents on Response to Toluene. Sens. Actuators B Chem. 2016, 230, 320–329. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Saini, R.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Reversible and Fast Responding Ppb Level Cl2 Sensor Based on Noncovalent Modified Carbon Nanotubes with Hexadecafluorinated Copper Phthalocyanine. Sens. Actuators B Chem. 2018, 255, 87–99. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, L.; Su, X.; Zhou, F.; Duan, G. Interfacial Self-Assembly of CoPc Thin Films with Their High Sensing Use as NO2 Sensors. Mater. Chem. Phys. 2019, 234, 94–101. [Google Scholar] [CrossRef]
- Roslan, N.A.; Abu Bakar, A.; Bawazeer, T.M.; Alsoufi, M.S.; Alsenany, N.; Abdul Majid, W.H.; Supangat, A. Enhancing the Performance of Vanadyl Phthalocyanine-Based Humidity Sensor by Varying the Thickness. Sens. Actuators B Chem. 2019, 279, 148–156. [Google Scholar] [CrossRef]
- Wang, X.; Ji, S.; Wang, H.; Yan, D. Room Temperature Nitrogen Dioxide Chemresistor Using Ultrathin Vanadyl-Phthalocyanine Film as Active Layer. Sens. Actuators B Chem. 2011, 160, 115–120. [Google Scholar] [CrossRef]
- Pan, L.; Jia, K.; Huang, Y.; Liu, X. Formation of Organometallic Microstructures via Self-Assembling of Carboxylated Zinc Phthalocyanines with Selective Adsorption and Visible Light-Driven Photodegradation of Cationic Dyes. J. Mater. Sci. 2018, 53, 492–505. [Google Scholar] [CrossRef]
- Pan, L.; Jia, K.; Shou, H.; Zhou, X.; Wang, P.; Liu, X. Unification of Molecular NIR Fluorescence and Aggregation-Induced Blue Emission via Novel Dendritic Zinc Phthalocyanines. J. Mater. Sci. 2017, 52, 3402–3418. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, F.; Yang, J.; Geng, Y.; Yan, D. Weak Epitaxy Growth Affording High-Mobility Thin Films of Disk-like Organic Semiconductors. Adv. Mater. 2007, 19, 2168–2171. [Google Scholar] [CrossRef]
- Cook, M.J.; Chambrier, I. Phthalocyanine Thin Films: Deposition and Structural Studies; Elsevier Inc.: Amsterdam, The Netherlands, 2003; Volume 17. [Google Scholar] [CrossRef]
- Schlettwein, D.; Graaf, H.; Meyer, J.-P.; Oekermann, T.; Jaeger, N.I. Molecular Interactions in Thin Films of Hexadecafluorophthalocyaninatozinc (F16PcZn) as Compared to Islands of N,N‘-Dimethylperylene-3,4,9,10-Biscarboximide (MePTCDI). J. Phys. Chem. B 1999, 103, 3078–3086. [Google Scholar] [CrossRef]
- Ziolo, R.F.; Griffiths, C.H.; Troup, J.M. Crystal Structure of Vanadyl Phthalocyanine, Phase II. J. Chem. Soc. Dalt. Trans. 1980, 575, 2300–2302. [Google Scholar] [CrossRef]
- Griffiths, C.H.; Walker, M.S.; Goldstein, P. Polymorphism in Vanadyl Phthalocyanine. Mol. Cryst. Liq. Cryst. 1976, 33, 149–170. [Google Scholar] [CrossRef]
- Pan, Y.L.; Wu, Y.J.; Chen, L.B.; Zhao, Y.Y.; Shen, Y.H.; Li, F.M.; Shen, S.Y.; Huang, D.H. Structure and Spectroscopic Characterization of Polycrystalline Vanadyl Phthalocyanine (VOPc) Films Fabricated by Vacuum Deposition. Appl. Phys. A Mater. Sci. Process. 1998, 66, 569–573. [Google Scholar] [CrossRef]
- Hoshi, H.; Hamamoto, K.; Yamada, T.; Ishikawa, K.; Takezoe, H.; Fukuda, A.; Fang, S.; Kohama, K.; Maruyama, Y. Thickness Dependence of the Epitaxial Structure of Vanadyl Phthalocyanine Film. Jpn. J. Appl. Phys. 1994, 33, L1555–L1558. [Google Scholar] [CrossRef]
- Minami, N.; Asai, M. Photocurrent Spectra of Phthalocyanine Films in Relation to Excited State Properties. Jpn. J. Appl. Phys. 1987, 26 Pt 1, 1754–1758. [Google Scholar] [CrossRef]
- Hiller, W.; Strähle, J.; Kobel, W.; Hanack, M. Polymorphie, Leitfähigkeit Und Kristallstrukturen von Oxo-Phthalocyaninato-Titan(IV). Z. Krist. New Cryst. Struct. 1982, 159, 173–183. [Google Scholar] [CrossRef]
- Bao, Z.; Lovinger, A.J.; Brown, J. New Air-Stable n-Channel Organs Thin Film Transistors. J. Am. Chem. Soc. 1998, 120, 207–208. [Google Scholar] [CrossRef]
- Shao, X.; Wang, S.; Li, X.; Su, Z.; Chen, Y.; Xiao, Y. Single Component P-, Ambipolar and n-Type OTFTs Based on Fluorinated Copper Phthalocyanines. Dye. Pigment. 2016, 132, 378–386. [Google Scholar] [CrossRef]
- Hesse, K.; Schlettwein, D. Spectroelectrochemical Investigations on the Reduction of Thin Films of Hexadecafluorophthalocyaninatozinc (F16PcZn). J. Electroanal. Chem. 1999, 476, 148–158. [Google Scholar] [CrossRef]
- Engel, M.K. Single-Crystal Structures of Phthalocyanine Complexes and Related Macrocycles; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 20. [Google Scholar] [CrossRef]
- Klyamer, D.; Sukhikh, A.; Gromilov, S.; Krasnov, P.; Basova, T. Fluorinated Metal Phthalocyanines: Interplay between Fluorination Degree, Films Orientation, and Ammonia Sensing Properties. Sensors 2018, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handa, M.; Suzuki, A.; Shoji, S.; Kasuga, K.; Sogabe, K. Spectral and Electrochemical Properties of Vanadyl Hexadecafluorophthalocyanine. Inorg. Chim. Acta 1995, 230, 41–44. [Google Scholar] [CrossRef]
- Schlettwein, D.; Tada, H.; Mashiko, S. Substrate-Induced Order and Multilayer Epitaxial Growth of Substituted Phthalocyanine Thin Films. Langmuir 2000, 16, 2872–2881. [Google Scholar] [CrossRef]
- Basova, T.V.; Kiselev, V.G.; Dubkov, I.S.; Latteyer, F.; Gromilov, S.A.; Peisert, H.; Chassè, T. Optical Spectroscopy and XRD Study of Molecular Orientation, Polymorphism, and Phase Transitions in Fluorinated Vanadyl Phthalocyanine Thin Films. J. Phys. Chem. C 2013, 117, 7097–7106. [Google Scholar] [CrossRef]
- Liu, B.; Cai, D.; Liu, Y.; Wang, D.; Wang, L.; Wang, Y.; Li, H.; Li, Q.; Wang, T. Improved Room-Temperature Hydrogen Sensing Performance of Directly Formed Pd/WO3 Nanocomposite. Sens. Actuators B Chem. 2014, 193, 28–34. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Zheng, B.; Geng, X.; Debliquy, M. Hydrogen Sensors Based on Noble Metal Doped Metal-Oxide Semiconductor: A Review. Int. J. Hydrogen Energy 2017, 42, 20386–20397. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Liu, Y.; Ma, J.; Li, X.; Du, Y.; Lu, G. Ordered Mesoporous Pd/SnO2 Synthesized by a Nanocasting Route for High Hydrogen Sensing Performance. Sens. Actuators B Chem. 2011, 160, 604–608. [Google Scholar] [CrossRef]
- Van Toan, N.; Viet Chien, N.; Van Duy, N.; Si Hong, H.; Nguyen, H.; Duc Hoa, N.; Van Hieu, N. Fabrication of Highly Sensitive and Selective H2 Gas Sensor Based on SnO2 Thin Film Sensitized with Microsized Pd Islands. J. Hazard. Mater. 2016, 301, 433–442. [Google Scholar] [CrossRef]
- Fardindoost, S.; Iraji zad, A.; Rahimi, F.; Ghasempour, R. Pd Doped WO3 Films Prepared by Sol–Gel Process for Hydrogen Sensing. Int. J. Hydrogen Energy 2010, 35, 854–860. [Google Scholar] [CrossRef]
- Jakubik, W.; Urbańczyk, M.; Maciak, E. Metal-Free Phthalocyanine and Palladium Sensor Structure with a Polyethylene Membrane for Hydrogen Detection in SAW Systems. Sens. Actuators B Chem. 2007, 127, 295–303. [Google Scholar] [CrossRef]
- Jakubik, W.P.; Urbańczyk, M.W.; Kochowski, S.; Bodzenta, J. Palladium and Phthalocyanine Bilayer Films for Hydrogen Detection in a Surface Acoustic Wave Sensor System. Sens. Actuators B Chem. 2003, 96, 321–328. [Google Scholar] [CrossRef]
- Jakubik, W.P.; Urbańczyk, M.W.; Kochowski, S.; Bodzenta, J. Bilayer Structure for Hydrogen Detection in a Surface Acoustic Wave Sensor System. Sens. Actuators B Chem. 2002, 82, 265–271. [Google Scholar] [CrossRef]
- Nikolaeva, N.S.; Parkhomenko, R.G.; Klyamer, D.D.; Shushanyan, A.D.; Asanov, I.P.; Morozova, N.B.; Basova, T.V. Bilayer Structures Based on Metal Phthalocyanine and Palladium Layers for Selective Hydrogen Detection. Int. J. Hydrogen Energy 2017, 42, 28640–28646. [Google Scholar] [CrossRef]
- Mckeown, N.B. Phthalocyanine Materials: Synthesis, Structure and Function; Dunn, B., Ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Sukhikh, A.S.; Basova, T.V.; Gromilov, S.A. Thin Layers XRD Study Technique on an Example of Cobalt Tetrafluoro Phthalocyanine. Acta Phys. Pol. A 2016, 130, 889–891. [Google Scholar] [CrossRef]
- Sukhikh, A.S.; Basova, T.V.; Gromilov, S.A. The Use of 2D Diffractometry Data for Oriented Samples in the Choice of a Unit Cell. J. Struct. Chem. 2017, 58, 953–963. [Google Scholar] [CrossRef]
- Chowdhury, A.; Biswas, B.; Majumder, M.; Sanyal, M.K.; Mallik, B. Studies on Phase Transformation and Molecular Orientation in Nanostructured Zinc Phthalocyanine Thin Films Annealed at Different Temperatures. Thin Solid Films 2012, 520, 6695–6704. [Google Scholar] [CrossRef]
- Karan, S.; Mallik, B. Effects of Annealing on the Morphology and Optical Property of Copper (II) Phthalocyanine Nanostructured Thin Films. Solid State Commun. 2007, 143, 289–294. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Trubin, S.V.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V.; Hassan, A.K. Tetrafluorosubstituted Metal Phthalocyanines: Interplay between Saturated Vapor Pressure and Crystal Structure. Cryst. Growth Des. 2020, 20, 1016–1024. [Google Scholar] [CrossRef]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of Sensing and Transduction for P-Type Semiconducting Metal Oxide Based Gas Sensors. J. Electroceramics 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Parkhomenko, R.G.; Sukhikh, A.S.; Klyamer, D.D.; Krasnov, P.O.; Gromilov, S.; Kadem, B.; Hassan, A.K.; Basova, T.V. Thin Films of Unsubstituted and Fluorinated Palladium Phthalocyanines: Structure and Sensor Response toward Ammonia and Hydrogen. J. Phys. Chem. C 2017, 121, 1200–1209. [Google Scholar] [CrossRef]
- Masui, M.; Sasahara, M.; Wada, T.; Takeuchi, M. Gas Sensitive Properties of Copper phthalocyanine Thin Films. Appl. Surf. Sci. 1996, 92, 643–646. [Google Scholar] [CrossRef]
- Hsieh, J.C.; Liu, C.J.; Ju, Y.H. Response Characteristics of Lead Phthalocyanine Gas Sensor: Effects of Film Thickness and Crystal Morphology. Thin Solid Films 1998, 322, 98–103. [Google Scholar] [CrossRef]
- Teschner, D.; Pestryakov, A.; Kleimenov, E.; Hävecker, M.; Bluhm, H.; Sauer, H.; Knop-Gericke, A.; Schlögl, R. High-Pressure X-Ray Photoelectron Spectroscopy of Palladium Model Hydrogenation Catalysts.: Part 1: Effect of Gas Ambient and Temperature. J. Catal. 2005, 230, 186–194. [Google Scholar] [CrossRef]
- Sohn, J.M.; Kang, S.K.; Woo, S.I. Catalytic Properties and Characterization of Pd Supported on Hexaaluminate in High Temperature Combustion. J. Mol. Catal. A Chem. 2002, 186, 135–144. [Google Scholar] [CrossRef]
- Aarnink, W.A.M.; Weishaupt, A.; van Silfhout, A. Angle-Resolved X-Ray Photoelectron Spectroscopy (ARXPS) and a Modified Levenberg-Marquardt Fit Procedure: A New Combination for Modeling Thin Layers. Appl. Surf. Sci. 1990, 45, 37–48. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Umar, A.; Tomar, M.; Gupta, V. Custom Designed Metal Anchored SnO2 Sensor for H2 Detection. Int. J. Hydrogen Energy 2017, 42, 4597–4609. [Google Scholar] [CrossRef]
- Chang, C.M.; Hon, M.H.; Leu, I.C. Improvement in CO Sensing Characteristics by Decorating ZnO Nanorod Arrays with Pd Nanoparticles and the Related Mechanisms. RSC Adv. 2012, 2, 2469–2475. [Google Scholar] [CrossRef]
- Chan, N.Y.; Zhao, M.; Huang, J.; Au, K.; Wong, M.H.; Yao, H.M.; Lu, W.; Chen, Y.; Ong, C.W.; Chan, H.L.W.; et al. Highly Sensitive Gas Sensor by the LaAlO3/SrTiO3 heterostructure with Pd Nanoparticle Surface Modulation. Adv. Mater. 2014, 26, 5962–5968. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, J.; Xie, F.; Wang, J.; Zhang, S.; Guo, X. Enhanced Performances of WO3-Based Hydrogen Sensors with an Amorphous SiO2 Layer Working at Low Temperatures. Solid State Ionics 2020, 347, 115274. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, K.; Yang, P.; Fu, X.; Wang, Z.; Yang, S.; Xiong, J.; Zhang, X.; Hu, Y.; Gu, H. Hydrogen Sensors Based on Pt-Decorated SnO2 Nanorods with Fast and Sensitive Room-Temperature Sensing Performance. J. Alloys Compd. 2019, 811, 152086. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Labat, F.; Ciofini, I.; Pauporté, T.; Adelung, R. Ultra-Sensitive and Selective Hydrogen Nanosensor with Fast Response at Room Temperature Based on a Single Pd/ZnO Nanowire. Sens. Actuators B Chem. 2018, 254, 1259–1270. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, J.; Wang, Y.; Sun, Y.; Ding, G. Temperature Dependent Response/Recovery Characteristics of Pd/Ni Thin Film Based Hydrogen Sensor. Sens. Actuators B Chem. 2019, 290, 544–550. [Google Scholar] [CrossRef]
- Raghu, S.; Santhosh, P.N.R.S. Nanostructured Palladium Modified Graphitic Carbon Nitride–High Performance Room Temperature Hydrogen Sensor. Int. J. Hydrogen Energy 2016, 41, 20779–20786. [Google Scholar] [CrossRef]










| Response/Recovery Time | NH3 (30 ppm) | H2 (300 ppm) |
|---|---|---|
| VOPc films | 15/65 | 18/100 |
| VOPcF4 films | 36/230 | 20/50 |
| Sensing Layer | C(H2) (ppm) | Response/ Recovery Time (s) | Temperature Range (°C) | Ref. |
|---|---|---|---|---|
| Pt-WO3 with an amorphous SiO2 layer | 3–150 | 18/349 (150 °C, 150 ppm) | 100–350 | [53] |
| Pt-decorated SnO2 nanorods (Pt/Sn ratio of 3.63%) | 100–1000 | 0.3/29.6 (room temperature (RT), 1000 ppm) | Room temperature | [54] |
| Pd/ZnO nanowire | 100 | 6.4/7.4 (RT, 100 ppm) | Room temperature | [55] |
| Pd/Ni film | 4000–20000 | 7/23 (75 °C, 20,000 ppm) | 25–100 | [56] |
| Pd/g-C3N4 | 1000–4000 | 88/- (30 °C, 4000 ppm)/ | 30–80 | [57] |
| VOPc films with Pd nanoparticles | 10–500 | 25/180 (100 ppm) | 80 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klyamer, D.; Sukhikh, A.; Nikolaeva, N.; Morozova, N.; Basova, T. Vanadyl Phthalocyanine Films and Their Hybrid Structures with Pd Nanoparticles: Structure and Sensing Properties. Sensors 2020, 20, 1893. https://doi.org/10.3390/s20071893
Klyamer D, Sukhikh A, Nikolaeva N, Morozova N, Basova T. Vanadyl Phthalocyanine Films and Their Hybrid Structures with Pd Nanoparticles: Structure and Sensing Properties. Sensors. 2020; 20(7):1893. https://doi.org/10.3390/s20071893
Chicago/Turabian StyleKlyamer, Darya, Aleksandr Sukhikh, Nataliya Nikolaeva, Natalya Morozova, and Tamara Basova. 2020. "Vanadyl Phthalocyanine Films and Their Hybrid Structures with Pd Nanoparticles: Structure and Sensing Properties" Sensors 20, no. 7: 1893. https://doi.org/10.3390/s20071893
APA StyleKlyamer, D., Sukhikh, A., Nikolaeva, N., Morozova, N., & Basova, T. (2020). Vanadyl Phthalocyanine Films and Their Hybrid Structures with Pd Nanoparticles: Structure and Sensing Properties. Sensors, 20(7), 1893. https://doi.org/10.3390/s20071893