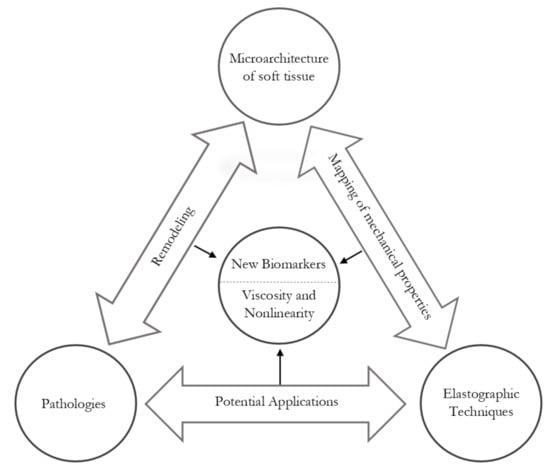
Why Are Viscosity and Nonlinearity Bound to Make an Impact in Clinical Elastographic Diagnosis?
Abstract
:1. Introduction
2. Mechanics of Soft Tissue
2.1. Soft Tissue Microstructure
2.2. Linear Elasticity
2.3. Viscoelasticity
2.4. Nonlinearity
3. Clinical Applications
3.1. Prostate
3.2. Breast
3.3. Liver
3.4. Labor Disorders
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| ECM | Extracellular Matrix |
| PGs | Proteoglycans |
| GAGs | Glycosaminoglycans |
| SMC | Smooth Muscle Cell |
| KV | Kelvin–Voigt |
| TOEC | Third-Order Elastic Constant |
| FOEC | Fourth-Order Elastic Constant |
| ARFI | Acoustic Radiation Force Impulse |
| pSWE | Point Shear Wave Elastography |
| TR-SWE | Transrectal Shear Wave Elastography |
| ROI | Region Of Interest |
| SDUV | Shear Wave Dispersion Ultrasound Vibrometry |
| KVFD | Kelvin-Voigt Fractional Derivative |
| DMA | Dynamic Mechanical Analysis |
| MRE | Magnetic Resonance Elastography |
| ELF | Enhanced Liver Fibrosis |
| WFUMB | World Federation of Ultrasound in Medicine and Biology |
| TE | Transient Elastography |
| SWE | Shear Wave Elastography |
| NAFLD | Non-alcoholic Fatty Liver Disease |
| SSI | Supersonic Shear Imaging |
| WHO | World Health Organization |
| SE | Static Elastography |
| SWEI | Shear Wave Elasticity Imaging |
| SWS | Shear Wave Speed |
| TWE | Torsional Wave Elastography |
| SPTB | Sponteneous Preterm Birth |
References
- Urban, M.W.; Chen, S.; Fatemi, M. A review of shearwave dispersion ultrasound vibrometry (SDUV) and its applications. Curr. Med. Imaging Rev. 2012, 8, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigrist, R.M.; Liau, J.; El Kaffas, A.; Chammas, M.C.; Willmann, J.K. Ultrasound elastography: Review of techniques and clinical applications. Theranostics 2017, 7, 1303. [Google Scholar] [CrossRef] [PubMed]
- Fruscalzo, A.; Londero, A.P.; Fröhlich, C.; Möllmann, U.; Schmitz, R. Quantitative Elastography for Cervical Stiffness Assessment during Pregnancy. BioMed Res. Int. 2014, 2014, 826535. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.C.; Feltovich, H.; Palmeri, M.L.; Dahl, J.J.; del Rio, A.M.; Hall, T.J. Estimation of shear wave speed in the human uterine cervix. Ultrasound Obstet. Gynecol. 2014, 43, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Bamber, J.; Cosgrove, D.; Dietrich, C.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.M.; D’onofrio, M.; Drakonaki, E.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. Eur. J. Ultrasound 2013, 34, 169–184. [Google Scholar] [CrossRef] [Green Version]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef] [Green Version]
- Săftoiu, A.; Gilja, O.H.; Sidhu, P.S.; Dietrich, C.F.; Cantisani, V.; Amy, D.; Bachmann-Nielsen, M.; Bob, F.; Bojunga, J.; Brock, M.; et al. The EFSUMB guidelines and recommendations for the clinical practice of elastography in non-hepatic applications: Update 2018. Ultraschall Med. Eur. J. Ultrasound 2019, 40, 425–453. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, D.; Piscaglia, F.; Bamber, J.; Bojunga, J.; Correas, J.M.; Gilja, O.; Klauser, A.; Sporea, I.; Calliada, F.; Cantisani, V.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. Eur. J. Ultrasound 2013, 34, 238–253. [Google Scholar]
- Riegler, J.; Labyed, Y.; Rosenzweig, S.; Javinal, V.; Castiglioni, A.; Dominguez, C.X.; Long, J.E.; Li, Q.; Sandoval, W.; Junttila, M.R.; et al. Tumor elastography and its association with collagen and the tumor microenvironment. Clin. Cancer Res. 2018, 24, 4455–4467. [Google Scholar] [CrossRef] [Green Version]
- Peralta, L.; Rus, G.; Bochud, N.; Molina, F. Mechanical assessment of cervical remodelling in pregnancy: Insight from a synthetic model. J. Biomech. 2015, 48, 1557–1565. [Google Scholar] [CrossRef]
- Carleton, J.B.; D’Amore, A.; Feaver, K.R.; Rodin, G.J.; Sacks, M.S. Geometric characterization and simulation of planar layered elastomeric fibrous biomaterials. Acta Biomater. 2015, 12, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Torres, J.; Faris, I.; Callejas, A. Histobiomechanical Remodeling of the Cervix during Pregnancy: Proposed Framework. Math. Probl. Eng. 2019, 2019, 5957432. [Google Scholar] [CrossRef]
- Holzapfel, G.A. Biomechanics of Soft Tissue. Handb. Mater. Behav. Model. 2001, 3, 1049–1063. [Google Scholar]
- Buehler, M.J. Nanomechanics of collagen fibrils under varying cross-link densities: Atomistic and continuum studies. J. Mech. Behav. Biomed. Mater. 2008, 1, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Canty, E.G.; Kadler, K.E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 2005, 118, 1341–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosini, S.; Pugh, N.; Bonna, A.M.; Hulmes, D.J.; Farndale, R.W.; Adams, J.C. Thrombospondin-1 promotes matrix homeostasis by interacting with collagen and lysyl oxidase precursors and collagen cross-linking sites. Sci. Signal. 2018, 11, eaar2566. [Google Scholar] [CrossRef] [Green Version]
- Elbjeirami, W.M.; Yonter, E.O.; Starcher, B.C.; West, J.L. Enhancing mechanical properties of tissue-engineered constructs via lysyl oxidase crosslinking activity. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 66, 513–521. [Google Scholar] [CrossRef]
- Ushiki, T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol. 2002, 65, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen fibril formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef]
- Herchenhan, A.; Uhlenbrock, F.; Eliasson, P.; Weis, M.; Eyre, D.; Kadler, K.E.; Magnusson, S.P.; Kjaer, M. Lysyl oxidase activity is required for ordered collagen fibrillogenesis by tendon cells. J. Biol. Chem. 2015, 290, 16440–16450. [Google Scholar] [CrossRef] [Green Version]
- Mäki, J.M.; Sormunen, R.; Lippo, S.; Kaarteenaho-Wiik, R.; Soininen, R.; Myllyharju, J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am. J. Pathol. 2005, 167, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.C.; Pinnell, S.R.; Martin, G.R. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry 1970, 9, 4486–4492. [Google Scholar] [CrossRef] [PubMed]
- Cronlund, A.L.; Smith, B.D.; Kagan, H.M. Binding of lysyl oxidase to fibrils of type I collagen. Connect. Tissue Res. 1985, 14, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Lanir, Y. A structural theory for the homogeneous biaxial stress-strain relationships in flat collagenous tissues. J. Biomech. 1979, 12, 423–436. [Google Scholar] [CrossRef]
- Chernoff, E.A.; Chernoff, D.A. Atomic force microscope images of collagen fibers. J. Vac. Sci. Technol. A Vac. Surfaces Films 1992, 10, 596–599. [Google Scholar] [CrossRef]
- Taroni, P.; Comelli, D.; Pifferi, A.; Torricelli, A.; Cubeddu, R. Absorption of collagen: Effects on the estimate of breast composition and related diagnostic implications. J. Biomed. Opt. 2007, 12, 014021. [Google Scholar] [CrossRef]
- Ambekar, R.; Lau, T.Y.; Walsh, M.; Bhargava, R.; Toussaint, K.C. Quantifying collagen structure in breast biopsies using second-harmonic generation imaging. Biomed. Opt. Express 2012, 3, 2021–2035. [Google Scholar] [CrossRef] [Green Version]
- Rojkind, M.; Ponce-Noyola, P. The extracellular matrix of the liver. Collagen Relat. Res. 1982, 2, 151–175. [Google Scholar] [CrossRef]
- Aycock, R.S.; Seyer, J.M. Collagens of normal and cirrhotic human liver. Connect. Tissue Res. 1989, 23, 19–31. [Google Scholar] [CrossRef]
- Oxlund, B.S.; Ørtoft, G.; Brüel, A.; Danielsen, C.C.; Bor, P.; Oxlund, H.; Uldbjerg, N. Collagen concentration and biomechanical properties of samples from the lower uterine cervix in relation to age and parity in non-pregnant women. Reprod. Biol. Endocrinol. 2010, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Sundtoft, I.; Langhoff-Roos, J.; Sandager, P.; Sommer, S.; Uldbjerg, N. Cervical collagen is reduced in non-pregnant women with a history of cervical insufficiency and a short cervix. Acta Obstet. Gynecol. Scand. 2017, 96, 984–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, K.M.; Paskaleva, A.; House, M.; Socrate, S. Mechanical and biochemical properties of human cervical tissue. Acta Biomater. 2008, 4, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Zhang, Y.; Zhang, M.B.; Li, Y.M.; Fei, X.; Song, Z.G. Tissue elasticity displayed by elastography and its correlation with the characteristics of collagen type I and type III in prostatic stroma. Asian J. Androl. 2014, 16, 305. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Fialkow, P.J.; Altman, L.K. The morphogenesis of elastic fibers. In Elastin and Elastic Tissue; Springer: Boston, MA, USA, 1977; pp. 7–17. [Google Scholar]
- Ferruzzi, J.; Collins, M.J.; Yeh, A.T.; Humphrey, J.D. Mechanical assessment of elastin integrity in fibrillin-1-deficient carotid arteries: Implications for Marfan syndrome. Cardiovasc. Res. 2011, 92, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Higuita Castro, N.; Hansford, D.J. Mechanical characterization of cells and tissues at the micro scale. Rev. Ing. Biomédica 2008, 2, 56–64. [Google Scholar]
- Rauscher, S.; Pomès, R. Structural disorder and protein elasticity. In Fuzziness; Springer: New York, NY, USA, 2012; pp. 159–183. [Google Scholar]
- Gosline, J.; Lillie, M.; Carrington, E.; Guerette, P.; Ortlepp, C.; Savage, K. Elastic proteins: Biological roles and mechanical properties. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2002, 357, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Lokshin, O.; Lanir, Y. Micro and macro rheology of planar tissues. Biomaterials 2009, 30, 3118–3127. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.E. Proteoglycan-fibrillar collagen interactions. Biochem. J. 1988, 252, 313. [Google Scholar] [CrossRef] [Green Version]
- Cöster, L. Structure and properties of dermatan sulphate proteoglycans. Biochem. Soc. Trans. 1991, 19, 866–868. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Engvall, E. Complexing of fibronectin glycosaminoglycans and collagen. Biochim. Biophys. Acta (BBA) Gen. Subj. 1980, 631, 350–358. [Google Scholar] [CrossRef]
- Cowin, S.C.; Doty, S.B. Tissue Mechanics; Springer Science & Business Media: New York, USA, 2007. [Google Scholar]
- Aristizabal, S. Anisotropic Shear Wave Elastography. In Ultrasound Elastography for Biomedical Applications and Medicine; John Wiley & Sons: West Sussex, UK, 2018; pp. 399–421. [Google Scholar]
- Depalle, B.; Qin, Z.; Shefelbine, S.J.; Buehler, M.J. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J. Mech. Behav. Biomed. Mater. 2015, 52, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, R.B.; Mulder, H.; Kovanen, V.; Magnusson, S.P. Fracture mechanics of collagen fibrils: Influence of natural cross-links. Biophys. J. 2013, 104, 2476–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peacock, C.J.; Kreplak, L. Nanomechanical mapping of single collagen fibrils under tension. Nanoscale 2019, 11, 14417–14425. [Google Scholar] [CrossRef] [PubMed]
- Schriefl, A.J.; Schmidt, T.; Balzani, D.; Sommer, G.; Holzapfel, G.A. Selective enzymatic removal of elastin and collagen from human abdominal aortas: Uniaxial mechanical response and constitutive modeling. Acta Biomater. 2015, 17, 125–136. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y. The orthotropic viscoelastic behavior of aortic elastin. Biomech. Model. Mechanobiol. 2011, 10, 613–625. [Google Scholar] [CrossRef]
- Sarvazyan, A.P.; Rudenko, O.V.; Swanson, S.D.; Fowlkes, J.; Emelianov, S.Y. Shear wave elasticity imaging: A new ultrasonic technology of medical diagnostics. Ultrasound Med. Biol. 1998, 24, 1419–1435. [Google Scholar] [CrossRef]
- Sarvazyan, A.P.; Urban, M.W.; Greenleaf, J.F. Acoustic waves in medical imaging and diagnostics. Ultrasound Med. Biol. 2013, 39, 1133–1146. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Li, H.; Zhang, Y. Understanding the viscoelastic behavior of collagen matrices through relaxation time distribution spectrum. Biomatter 2013, 3, e24651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.L.; Kahn, H.; Ballarini, R.; Eppell, S.J. Viscoelastic properties of isolated collagen fibrils. Biophys. J. 2011, 100, 3008–3015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowin, S.C. Deviatoric and hydrostatic mode interaction in hard and soft tissue. J. Biomech. 1990, 23, 11–14. [Google Scholar] [CrossRef]
- Holzapfel, G.A.; Gasser, T.C.; Ogden, R.W. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. Phys. Sci. Solids 2000, 61, 1–48. [Google Scholar]
- Duck, F.A. Physical Properties of Tissues: A Comprehensive Reference Book; Academic Press: London, UK, 2013. [Google Scholar]
- Nightingale, K.R.; Rouze, N.C.; Rosenzweig, S.J.; Wang, M.H.; Abdelmalek, M.F.; Guy, C.D.; Palmeri, M.L. Derivation and analysis of viscoelastic properties in human liver: Impact of frequency on fibrosis and steatosis staging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trutna, C.A.; Rouze, N.C.; Palmeri, M.L.; Nightingale, K.R. Measurement of Viscoelastic Material Model Parameters using Fractional Derivative Group Shear Wave Speeds in Simulation and Phantom Data. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 67, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, P.; Chen, S.; Urban, M.W.; McGough, R.J. Shear elasticity and shear viscosity imaging in viscoelastic phantoms. J. Acoust. Soc. Am. 2017, 141, 3720–3721. [Google Scholar] [CrossRef]
- Amador, C.; Kinnick, R.R.; Urban, M.W.; Fatemi, M.; Greenleaf, J.F. Viscoelastic tissue mimicking phantom validation study with shear wave elasticity imaging and viscoelastic spectroscopy. In Proceedings of the 2015 IEEE International Ultrasonics Symposium (IUS), Taipei, Taiwan, 21–24 October 2015; pp. 1–4. [Google Scholar]
- Qiang, B.; Brigham, J.C.; Aristizabal, S.; Greenleaf, J.F.; Zhang, X.; Urban, M.W. Modeling transversely isotropic, viscoelastic, incompressible tissue-like materials with application in ultrasound shear wave elastography. Phys. Med. Biol. 2015, 60, 1289. [Google Scholar] [CrossRef] [Green Version]
- Gennisson, J.L.; Aristizabal, S. Nonlinear Shear Elasticity. In Ultrasound Elastography for Biomedical Applications and Medicine; John Wiley & Sons: West Sussex, UK, 2018; pp. 451–469. [Google Scholar]
- Hu, Y.; Suo, Z. Viscoelasticity and poroelasticity in elastomeric gels. Acta Mech. Solida Sin. 2012, 25, 441–458. [Google Scholar] [CrossRef]
- Wang, Q.M.; Mohan, A.C.; Oyen, M.L.; Zhao, X.H. Separating viscoelasticity and poroelasticity of gels with different length and time scales. Acta Mech. Sin. 2014, 30, 20–27. [Google Scholar] [CrossRef]
- Al Mayah, A. Biomechanics of Soft Tissues: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Muir, H. Proteoglycans as organizers of the intercellular matrix. Biochem. Soc. Trans. 1983, 11, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Soltz, M.A.; Ateshian, G.A. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J. Biomech. 1998, 31, 927–934. [Google Scholar] [CrossRef]
- Cardoso, L.; Cowin, S.C. Role of structural anisotropy of biological tissues in poroelastic wave propagation. Mech. Mater. 2012, 44, 174–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brum, J.; Bernal, M.; Gennisson, J.; Tanter, M. In vivo evaluation of the elastic anisotropy of the human Achilles tendon using shear wave dispersion analysis. Phys. Med. Biol. 2014, 59, 505. [Google Scholar] [CrossRef] [PubMed]
- Peralta, L.; Rus, G.; Bochud, N.; Molina, F. Assessing viscoelasticity of shear wave propagation in cervical tissue by multiscale computational simulation. J. Biomech. 2015, 48, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Shiina, T.; Ueno, E. Quantitative assessment and imaging of viscoelastic properties of soft tissue. In Proceedings of the 2002 IEEE Ultrasonics Symposium, Munich, Germany, 8–11 October 2002; Volume 2, pp. 1885–1889. [Google Scholar]
- Sack, I.; Jöhrens, K.; Würfel, J.; Braun, J. Structure-sensitive elastography: On the viscoelastic powerlaw behavior of in vivo human tissue in health and disease. Soft Matter 2013, 9, 5672–5680. [Google Scholar] [CrossRef]
- Gautieri, A.; Vesentini, S.; Redaelli, A.; Ballarini, R. Modeling and measuring visco-elastic properties: From collagen molecules to collagen fibrils. Int. J. Non-Linear Mech. 2013, 56, 25–33. [Google Scholar] [CrossRef]
- García, A.; Martínez, M.; Pena, E. Viscoelastic properties of the passive mechanical behavior of the porcine carotid artery: Influence of proximal and distal positions. Biorheology 2012, 49, 271–288. [Google Scholar] [CrossRef]
- Soby, L.; Jamieson, A.; Blackwell, J.; Choi, H.; Rosenberg, L. Viscoelastic and rheological properties of concentrated solutions of proteoglycan subunit and proteoglycan aggregate. Biopolym. Orig. Res. Biomol. 1990, 29, 1587–1592. [Google Scholar] [CrossRef]
- Sridhar, M.; Liu, J.; Insana, M. Viscoelasticity imaging using ultrasound: Parameters and error analysis. Phys. Med. Biol. 2007, 52, 2425. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, L.; Tredup, C.; Gubbiotti, M.A.; Iozzo, R.V. Proteoglycan neofunctions: Regulation of inflammation and autophagy in cancer biology. FEBS J. 2017, 284, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Frey, H.; Schroeder, N.; Manon-Jensen, T.; Iozzo, R.V.; Schaefer, L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013, 280, 2165–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losa, G.A.; Alini, M. Sulfated proteoglycans in the extracellular matrix of human breast tissues with infiltrating carcinoma. Int. J. Cancer 1993, 54, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Mendez, I.M.; Palmeri, M.L.; Drehfal, L.C.; Guerrero, Q.W.; Simmons, H.; Feltovich, H.; Hall, T.J. Assessment of structural heterogeneity and viscosity in the cervix using shear wave elasticity imaging: Initial results from a rhesus macaque model. Ultrasound Med. Biol. 2017, 43, 790–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, L.K.; Oxlund, H.; Uldbjerg, N.; Forman, A. In vitro analysis of muscular contractile ability and passive biomechanical properties of uterine cervical samples from nonpregnant women. Obstet. Gynecol. 1991, 77, 772–776. [Google Scholar] [PubMed]
- Yao, W.; Yoshida, K.; Fernandez, M.; Vink, J.; Wapner, R.J.; Ananth, C.V.; Oyen, M.L.; Myers, K.M. Measuring the compressive viscoelastic mechanical properties of human cervical tissue using indentation. J. Mech. Behav. Biomed. Mater. 2014, 34, 18–26. [Google Scholar] [CrossRef]
- Ghatak, S.; Maytin, E.V.; Mack, J.A.; Hascall, V.C.; Atanelishvili, I.; Moreno Rodriguez, R.; Markwald, R.R.; Misra, S. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int. J. Cell Biol. 2015, 2015, 834893. [Google Scholar] [CrossRef] [Green Version]
- Armentano, R.L.; Barra, J.G.; Santana, D.B.; Pessana, F.M.; Graf, S.; Craiem, D.; Brandani, L.M.; Baglivo, H.P.; Sanchez, R.A. Smart damping modulation of carotid wall energetics in human hypertension: Effects of angiotensin-converting enzyme inhibition. Hypertension 2006, 47, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Vink, J.Y.; Qin, S.; Brock, C.O.; Zork, N.M.; Feltovich, H.M.; Chen, X.; Urie, P.; Myers, K.M.; Hall, T.J.; Wapner, R.; et al. A new paradigm for the role of smooth muscle cells in the human cervix. Am. J. Obstet. Gynecol. 2016, 215, 478.e1. [Google Scholar] [CrossRef] [Green Version]
- Yamaoka, K.; Nouchi, T.; Marumo, F.; Sato, C. α-Smooth-muscle actin expression in normal and fibrotic human livers. Dig. Dis. Sci. 1993, 38, 1473–1479. [Google Scholar] [CrossRef]
- Langewouters, G.; Wesseling, K.; Goedhard, W. The pressure dependent dynamic elasticity of 35 thoracic and 16 abdominal human aortas in vitro described by a five component model. J. Biomech. 1985, 18, 613–620. [Google Scholar] [CrossRef]
- Kumar, V.; Denis, M.; Gregory, A.; Bayat, M.; Mehrmohammadi, M.; Fazzio, R.; Fatemi, M.; Alizad, A. Viscoelastic parameters as discriminators of breast masses: Initial human study results. PLoS ONE 2018, 13, e0205717. [Google Scholar] [CrossRef] [Green Version]
- Nabavizadeh, A.; Bayat, M.; Kumar, V.; Gregory, A.; Webb, J.; Alizad, A.; Fatemi, M. Viscoelastic biomarker for differentiation of benign and malignant breast lesion in ultra-low frequency range. Sci. Rep. 2019, 9, 5737. [Google Scholar] [CrossRef] [Green Version]
- Sinkus, R.; Siegmann, K.; Xydeas, T.; Tanter, M.; Claussen, C.; Fink, M. MR elastography of breast lesions: Understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2007, 58, 1135–1144. [Google Scholar] [CrossRef]
- Balleyguier, C.; Canale, S.; Hassen, W.B.; Vielh, P.; Bayou, E.; Mathieu, M.; Uzan, C.; Bourgier, C.; Dromain, C. Breast elasticity: Principles, technique, results: An update and overview of commercially available software. Eur. J. Radiol. 2013, 82, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, M.L.; Nightingale, K.R. What challenges must be overcome before ultrasound elasticity imaging is ready for the clinic? Imaging Med. 2011, 3, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aristizabal, S.; Carrascal, C.A.; Nenadic, I.Z.; Greenleaf, J.F.; Urban, M.W. Application of Acoustoelasticity to Evaluate Nonlinear Modulus inEx VivoKidneys. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 188–200. [Google Scholar] [CrossRef]
- Schregel, K.; née Tysiak, E.W.; Garteiser, P.; Gemeinhardt, I.; Prozorovski, T.; Aktas, O.; Merz, H.; Petersen, D.; Wuerfel, J.; Sinkus, R. Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance elastography. Proc. Natl. Acad. Sci. USA 2012, 109, 6650–6655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streitberger, K.J.; Sack, I.; Krefting, D.; Pfüller, C.; Braun, J.; Paul, F.; Wuerfel, J. Brain viscoelasticity alteration in chronic-progressive multiple sclerosis. PLoS ONE 2012, 7, e29888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sack, I.; Beierbach, B.; Wuerfel, J.; Klatt, D.; Hamhaber, U.; Papazoglou, S.; Martus, P.; Braun, J. The impact of aging and gender on brain viscoelasticity. Neuroimage 2009, 46, 652–657. [Google Scholar] [CrossRef]
- Chen, S.; Urban, M.W.; Pislaru, C.; Kinnick, R.; Zheng, Y.; Yao, A.; Greenleaf, J.F. Shearwave dispersion ultrasound vibrometry (SDUV) for measuring tissue elasticity and viscosity. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Sanchez, W.; Callstrom, M.R.; Gorman, B.; Lewis, J.T.; Sanderson, S.O.; Greenleaf, J.F.; Xie, H.; Shi, Y.; Pashley, M.; et al. Assessment of liver viscoelasticity by using shear waves induced by ultrasound radiation force. Radiology 2013, 266, 964–970. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Zhang, X.; Shen, Y.; Zheng, Y.; Guo, Y.; Zhu, Y.; Diao, X.; Wang, T.; Chen, S.; Chen, X. Model-dependent and model-independent approaches for evaluating hepatic fibrosis in rat liver using shearwave dispersion ultrasound vibrometry. Med. Eng. Phys. 2017, 39, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Mitri, F.G.; Urban, M.W.; Fatemi, M.; Member, S.; Greenleaf, J.F.; Fellow, L. Shear Wave Dispersion Ultrasonic Vibrometry for Measuring Prostate Shear Stiffness and Viscosity: An In Vitro Pilot Study. IEEE Trans. Biomed. Eng. 2011, 58, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Nigwekar, P.; Castaneda, B.; Hoyt, K.; Joseph, J.V.; di Sant’Agnese, A.; Messing, E.M.; Strang, J.G.; Rubens, D.J.; Parker, K.J. Quantitative characterization of viscoelastic properties of human prostate correlated with histology. Ultrasound Med. Biol. 2008, 34, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Sinkus, R.; Tanter, M.; Xydeas, T.; Catheline, S.; Bercoff, J.; Fink, M. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magn. Reson. Imaging 2005, 23, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Sinkus, R.; Tanter, M.; Catheline, S.; Lorenzen, J.; Kuhl, C.; Sondermann, E.; Fink, M. Imaging anisotropic and viscous properties of breast tissue by magnetic resonance-elastography. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2005, 53, 372–387. [Google Scholar] [CrossRef]
- Nenadic, I.Z.; Qiang, B.; Urban, M.W.; Zhao, H.; Sanchez, W.; Greenleaf, J.F.; Chen, S. Attenuation measuring ultrasound shearwave elastography and in vivo application in post-transplant liver patients. Phys. Med. Biol. 2016, 62, 484. [Google Scholar] [CrossRef] [Green Version]
- Klatt, D.; Hamhaber, U.; Asbach, P.; Braun, J.; Sack, I. Noninvasive assessment of the rheological behavior of human organs using multifrequency MR elastography: A study of brain and liver viscoelasticity. Phys. Med. Biol. 2007, 52, 7281–7294. [Google Scholar] [CrossRef]
- Asbach, P.; Klatt, D.; Hamhaber, U.; Braun, J.; Somasundaram, R.; Hamm, B.; Sack, I. Assessment of liver viscoelasticity using multifrequency MR elastography. Magn. Reson. Med. 2008, 60, 373–379. [Google Scholar] [CrossRef]
- Li, S.; Chen, M.; Wang, W.; Zhao, W.; Wang, J.; Zhao, X.; Zhou, C. A feasibility study of MR elastography in the diagnosis of prostate cancer at 3.0 T. Acta Radiol. 2011, 52, 354–358. [Google Scholar] [CrossRef]
- Sugimoto, K.; Moriyasu, F.; Oshiro, H.; Takeuchi, H.; Yoshimasu, Y.; Kasai, Y.; Itoi, T. Clinical utilization of shear wave dispersion imaging in the diffuse liver disease. Ultrasonography 2019, 39, 3. [Google Scholar] [CrossRef] [Green Version]
- Deffieux, T.; Gennisson, J.L.; Bousquet, L.; Corouge, M.; Cosconea, S.; Amroun, D.; Tripon, S.; Terris, B.; Mallet, V.; Sogni, P.; et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J. Hepatol. 2015, 62, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Callejas, A.; Gomez, A.; Melchor, J.; Riveiro, M.; Massó, P.; Torres, J.; López-López, M.; Rus, G. Performance study of a torsional wave sensor and cervical tissue characterization. Sensors 2017, 17, 2078. [Google Scholar] [CrossRef] [PubMed]
- Susilo, M.E.; Paten, J.A.; Sander, E.A.; Nguyen, T.D.; Ruberti, J.W. Collagen network strengthening following cyclic tensile loading. Interface Focus 2016, 6, 20150088. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Burcher, M.; Noble, J.A. Non-invasive measurement of biomechanical properties of in vivo soft tissues. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Tokyo, Japan, 25–28 September 2002; pp. 208–215. [Google Scholar]
- Serra-Aguila, A.; Puigoriol-Forcada, J.; Reyes, G.; Menacho, J. Viscoelastic models revisited: Characteristics and interconversion formulas for generalized Kelvin–Voigt and Maxwell models. Acta Mech. Sin. 2019, 35, 1191–1209. [Google Scholar] [CrossRef]
- Schmitt, C.; Henni, A.H.; Cloutier, G. Characterization of blood clot viscoelasticity by dynamic ultrasound elastography and modeling of the rheological behavior. J. Biomech. 2011, 44, 622–629. [Google Scholar] [CrossRef]
- Fung, Y.C. Biomechanics: Mechanical Properties of Living Tissues; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Parker, K.; Szabo, T.; Holm, S. Towards a consensus on rheological models for elastography in soft tissues. Phys. Med. Biol. 2019, 64, 215012. [Google Scholar] [CrossRef] [Green Version]
- Koeller, R. Applications of fractional calculus to the theory of viscoelasticity. J. Appl. Mech. 1984, 51, 299–307. [Google Scholar] [CrossRef]
- Rus, G. Nature of acoustic nonlinear radiation stress. Appl. Phys. Lett. 2014, 105, 121904. [Google Scholar] [CrossRef] [Green Version]
- Gomez, A. Transurethral Shear Wave Elastography for Prostate Cancer. Ph.D. Thesis, University College London, London, UK, 2018. [Google Scholar]
- Orescanin, M.; Wang, Y.; Insana, M.F. 3-D FDTD simulation of shear waves for evaluation of complex modulus imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 389–398. [Google Scholar] [CrossRef]
- Bercoff, J.; Tanter, M.; Muller, M.; Fink, M. The role of viscosity in the impulse diffraction field of elastic waves induced by the acoustic radiation force. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 1523–1536. [Google Scholar] [CrossRef]
- Chen, L.H.; Ng, S.P.; Yu, W.; Zhou, J.; Wan, K.F. A study of breast motion using non-linear dynamic FE analysis. Ergonomics 2013, 56, 868–878. [Google Scholar] [CrossRef]
- Salameh, N.; Peeters, F.; Sinkus, R.; Abarca-Quinones, J.; Annet, L.; Ter Beek, L.C.; Leclercq, I.; Van Beers, B.E. Hepatic viscoelastic parameters measured with MR elastography: Correlations with quantitative analysis of liver fibrosis in the rat. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2007, 26, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Palacio-Torralba, J.; Hammer, S.; Good, D.W.; McNeill, S.A.; Stewart, G.D.; Reuben, R.L.; Chen, Y. Quantitative diagnostics of soft tissue through viscoelastic characterization using time-based instrumented palpation. J. Mech. Behav. Biomed. Mater. 2015, 41, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vappou, J.; Maleke, C.; Konofagou, E.E. Quantitative viscoelastic parameters measured by harmonic motion imaging. Phys. Med. Biol. 2009, 54, 3579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Castaneda, B.; Wu, Z.; Nigwekar, P.; Joseph, J.V.; Rubens, D.J.; Parker, K.J. Congruence of imaging estimators and mechanical measurements of viscoelastic properties of soft tissues. Ultrasound Med. Biol. 2007, 33, 1617–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, M.; Moussu, M.A.; Chayer, B.; Destrempes, F.; Gesnik, M.; Allard, L.; Tang, A.; Cloutier, G. Reconstruction of Viscosity Maps in Ultrasound Shear Wave Elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Lanir, Y. Multi-scale structural modeling of soft tissues mechanics and mechanobiology. J. Elast. 2017, 129, 7–48. [Google Scholar] [CrossRef]
- Pritchard, R.H.; Huang, Y.Y.S.; Terentjev, E.M. Mechanics of biological networks: From the cell cytoskeleton to connective tissue. Soft Matter 2014, 10, 1864–1884. [Google Scholar] [CrossRef]
- Myers, K.M.; Feltovich, H.; Mazza, E.; Vink, J.; Bajka, M.; Wapner, R.J.; Hall, T.J.; House, M. The mechanical role of the cervix in pregnancy. J. Biomech. 2015, 48, 1511–1523. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, R.; Melchor, J. Nonlinear Classical Elasticity Model for Materials with Fluid and Matrix Phases. Math. Probl. Eng. 2018, 2018, 5049104. [Google Scholar] [CrossRef]
- Goenezen, S.; Dord, J.F.; Sink, Z.; Barbone, P.E.; Jiang, J.; Hall, T.J.; Oberai, A.A. Linear and nonlinear elastic modulus imaging: An application to breast cancer diagnosis. IEEE Trans. Med. Imaging 2012, 31, 1628–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landau, L.D.; Lifshitz, E.M. Course of Theoretical Physics Volume 7: Theory and Elasticity; Pergamon Press: Oxford, UK, 1959. [Google Scholar]
- Murnaghan, F. The compressibility of media under extreme pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasser, T.C.; Ogden, R.W.; Holzapfel, G.A. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 2005, 3, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Ogden, R.W.; Holzapfel, G.A. Mechanics of Biological Tissue; Springer: Berlin, Germany, 2006. [Google Scholar]
- Ovenden, N.; Walsh, C. Fundamentals of Physiological Solid Mechanics. In Fluid and Solid Mechanics; World Scientific: London, UK, 2016; pp. 169–217. [Google Scholar]
- Truesdell, C.; Noll, W. The non-linear field theories of mechanics. In The Non-Linear Field Theories of Mechanics; Springer: Berlin, Germany, 2004; pp. 1–579. [Google Scholar]
- Zabolotskaya, E.A.; Hamilton, M.F.; Ilinskii, Y.A.; Meegan, G.D. Modeling of nonlinear shear waves in soft solids. J. Acoust. Soc. Am. 2004, 116, 2807–2813. [Google Scholar] [CrossRef]
- Bernal, M.M.; Chammings, F.; Couade, M.; Bercoff, J.; Tanter, M.; luc Gennisson, J. In Vivo Quantification of the Nonlinear Shear Modulus in Breast Lesions: Feasibility Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Melchor, J.; Parnell, W.; Bochud, N.; Peralta, L.; Rus, G. Damage prediction via nonlinear ultrasound: A micro-mechanical approach. Ultrasonics 2019, 93, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Sack, I.; Mcgowan, C.K.; Samani, A.; Luginbuhl, C.; Oakden, W.; Plewes, D.B. Observation of nonlinear shear wave propagation using magnetic resonance elastography. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2004, 52, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Melchor, J. Mechanics of Nonlinear Ultrasound in Tissue. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2016. [Google Scholar]
- Naranjo-Pérez, J.; Riveiro, M.; Callejas, A.; Rus, G.; Melchor, J. Nonlinear torsional wave propagation in cylindrical coordinates to assess biomechanical parameters. J. Sound Vib. 2019, 445, 103–116. [Google Scholar] [CrossRef]
- Zahedmanesh, H.; Lally, C. A multiscale mechanobiological modelling framework using agent-based models and finite element analysis: Application to vascular tissue engineering. Biomech. Model. Mechanobiol. 2012, 11, 363–377. [Google Scholar] [CrossRef]
- Maceri, F.; Marino, M.; Vairo, G. A unified multiscale mechanical model for soft collagenous tissues with regular fiber arrangement. J. Biomech. 2010, 43, 355–363. [Google Scholar] [CrossRef]
- Hamilton, M.F.; Blackstock, D.T. (Eds.) Nonlinear Acoustics; Academic Press: San Diego, CA, USA, 1998; Volume 237. [Google Scholar]
- Rushchitsky, J.; Simchuk, Y.V. Quadratic nonlinear torsional hyperelastic waves in isotropic cylinders: Primary analysis of evolution. Int. Appl. Mech. 2008, 44, 304–312. [Google Scholar] [CrossRef]
- Latorre-Ossa, H.; Gennisson, J.L.; De Brosses, E.; Tanter, M. Quantitative imaging of nonlinear shear modulus by combining static elastography and shear wave elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Memo, R.; Schaub, C.R. Shear wave ultrasound elastography of the prostate: Initial results. Ultrasound Q. 2012, 28, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, L.; Tanter, M.; Catheline, S.; Fink, M. Shear modulus imaging with 2-D transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 426–435. [Google Scholar] [CrossRef]
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, G.; Qian, L.X.; Liang, S.; Destrade, M.; Cao, Y. Measuring the linear and nonlinear elastic properties of brain tissue with shear waves and inverse analysis. Biomech. Model. Mechanobiol. 2015, 14, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Jacob, X.; Catheline, S.; Gennisson, J.L.; Barrière, C.; Royer, D.; Fink, M. Nonlinear shear wave interaction in soft solids. J. Acoust. Soc. Am. 2007, 122, 1917–1926. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, G.Y.; Qian, L.X.; Hu, X.D.; Liu, D.; Liang, S.; Cao, Y. Characterization of the nonlinear elastic properties of soft tissues using the supersonic shear imaging (SSI) technique: Inverse method, ex vivo and in vivo experiments. Med. Image Anal. 2015, 20, 97–111. [Google Scholar] [CrossRef]
- Parker, K.J.; Doyley, M.M.; Rubens, D.J. Imaging the elastic properties of tissue: The 20 year perspective. Phys. Med. Biol. 2011, 56 1, R1–R29. [Google Scholar] [CrossRef]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom. Radiol. 2018, 43, 773–785. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Abdellaoui, A.; Iyengar, S.; Freeman, S. Imaging in prostate cancer. Futur. Oncol. 2011, 7, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Cosgrove, D.; Brock, M.; Cantisani, V.; Correas, J.M.; Postema, A.W.; Salomon, G.; Tsutsumi, M.; Xu, H.X.; Dietrich, C.F. WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: Part 5. Prostate. Ultrasound Med. Biol. 2017, 43, 27–48. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.; Madden, J.; Foo, W.C.; Palmeri, M.L.; Mouraviev, V.; Polascik, T.J.; Nightingale, K.R. Acoustic radiation force impulse imaging of human prostates ex vivo. Ultrasound Med. Biol. 2010, 36, 576–588. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.; Polascik, T.J.; Foo, W.C.; Rosenzweig, S.; Palmeri, M.L.; Madden, J.; Nightingale, K.R. Acoustic Radiation Force Impulse Imaging of Human Prostates: Initial In Vivo Demonstration. Ultrasound Med. Biol. 2012, 38, 50–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correas, J.M.; Tissier, A.M.; Khairoune, A.; Vassiliu, V.; Méjean, A.; Hélénon, O.; Memo, R.; Barr, R.G. Prostate Cancer: Diagnostic Performance of Real-time Shear-Wave Elastography. Radiology 2015, 275, 280–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correas, J.; Drakonakis, E.; Isidori, A.M.; Hélénon, O.; Pozza, C.; Cantisani, V.; Di Leo, N.; Maghella, F.; Rubini, a.; Drudi, F.; et al. Update on ultrasound elastography: Miscellanea. Prostate, testicle, musculo-skeletal. Eur. J. Radiol. 2013, 82, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fu, S.; Zhang, Y.; Tang, J.; Zhou, Y. Elastic modulus of the prostate: A new non-invasive feature to diagnose bladder outlet obstruction in patients with benign prostatic hyperplasia. Ultrasound Med. Biol. 2013, 40, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Shear wave elastography for detection of prostate cancer: A preliminary study. Korean J. Radiol. 2014, 15, 346–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correas, J.M.; Khairoune, A.; Tissier, A.M.; Vassiliu, V. Trans-rectal quantitative Shear Wave Elastrography: Application to prostate cancer—A feasibility study. In Proceedings of the European Congress of Radiology, Vienna, Austria, 3–7 March 2011. [Google Scholar]
- Boehm, K.; Salomon, G.; Beyer, B.; Schiffmann, J.; Simonis, K.; Graefen, M.; Budaeus, L. Shear Wave Elastography for Localization of Prostate Cancer Lesions and Assessment of Elasticity Thresholds: Implications for Targeted Biopsies and Active Surveillance Protocols. J. Urol. 2015, 193, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Kim, S.Y.; Lee, M.S.; Cho, J.Y.; Kim, S.H. Shear wave elastography assessment in the prostate: An intraobserver reproducibility study. Clin. Imaging 2015, 39, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Bercoff, J.; Athanasiou, A.; Deffieux, T.; Gennisson, J.L.; Montaldo, G.; Muller, M.; Tardivon, A.; Fink, M. Quantitative assessment of breast lesion viscoelasticity: Initial clinical results using supersonic shear imaging. Ultrasound Med. Biol. 2008, 34, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Madden, J.; Foo, W.C.; Mouraviev, V.; Polascik, T.J.; Palmeri, M.L.; Nightingale, K.R. Characterizing Stiffness of Human Prostates Using Acoustic Radiation Force. Ultrason. Imaging 2010, 32, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Ji, P.; Mao, H.; Hu, J. A comparison of virtual touch tissue quantification and digital rectal examination for discrimination between prostate cancer and benign prostatic hyperplasia. Radiol. Oncol. 2012, 46, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyt, K.; Castaneda, B.; Zhang, M.; Nigwekar, P.; Anthony, P.; Agnese, S.; Joseph, J.V.; Strang, J.; Rubens, D.J.; Parker, K.J. Tissue elasticity properties as biomarkers for prostate cancer. Cancer Biomark. 2008, 4, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Chopra, R.; Arani, A.; Huang, Y.; Musquera, M.; Wachsmuth, J.; Bronskill, M.; Plewes, D. In vivo MR elastography of the prostate gland using a transurethral actuator. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2009, 62, 665–671. [Google Scholar] [CrossRef]
- Arani, A.; Plewes, D.; Chopra, R. Transurethral prostate magnetic resonance elastography: Prospective imaging requirements. Magn. Reson. Med. 2011, 65, 340–349. [Google Scholar] [CrossRef]
- Reiter, R.; Majumdar, S.; Kearney, S.; Kajdacsy-Balla, A.; Macias, V.; Crivellaro, S.; Caldwell, B.; Abern, M.; Royston, T.J.; Klatt, D. Prostate cancer assessment using MR elastography of fresh prostatectomy specimens at 9.4 T. Magn. Reson. Med. 2019, 84, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Orel, S.G.; Kay, N.; Reynolds, C.; Sullivan, D.C. BI-RADS categorization as a predictor of malignancy. Radiology 1999, 211, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Leong, L.; Sim, L.; Lee, Y.; Ng, F.; Wan, C.; Fook-Chong, S.; Jara-Lazaro, A.; Tan, P. A prospective study to compare the diagnostic performance of breast elastography versus conventional breast ultrasound. Clin. Radiol. 2010, 65, 887–894. [Google Scholar] [CrossRef]
- Goddi, A.; Bonardi, M.; Alessi, S. Breast elastography: A literature review. J. Ultrasound 2012, 15, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; McCarron, P.; Parkin, D.M. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004, 6, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culpan, A.M. Breast Elastography. Imaging & Therapy Practice. 2016. 30. Available online: https://search.proquest.com/openview/a5377f86d950c0ca1132920c07c00430/1.pdf?pq-origsite=gscholar&cbl=46803 (accessed on 25 March 2020).
- Barr, R.G.; Nakashima, K.; Amy, D.; Cosgrove, D.; Farrokh, A.; Schafer, F.; Bamber, J.C.; Castera, L.; Choi, B.I.; Chou, Y.H.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 2: Breast. Ultrasound Med. Biol. 2015, 41, 1148–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, R.G.; Destounis, S.; Lackey, L.B.; Svensson, W.E.; Balleyguier, C.; Smith, C. Evaluation of breast lesions using sonographic elasticity imaging: A multicenter trial. J. Ultrasound Med. 2012, 31, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, D.W.; Fang, Y.X.; Song, Y.J.; Deng, Z.J.; Gao, J.; Xie, Y.; Yin, T.S.; Ying, L.; Tang, K.F. Performance of shear wave elastography for differentiation of benign and malignant solid breast masses. PLoS ONE 2013, 8, e76322. [Google Scholar] [CrossRef]
- D’Orsi, C.; Bassett, L.; Feig, S. Breast imaging reporting and data system (BI-RADS). In Breast Imaging Atlas, 4th ed.; American College of Radiology: Reston, VA, USA, 1998. [Google Scholar]
- Chen, L.; He, J.; Liu, G.; Shao, K.; Zhou, M.; Li, B.; Chen, X. Diagnostic performances of shear-wave elastography for identification of malignant breast lesions: A meta-analysis. Jpn. J. Radiol. 2014, 32, 592–599. [Google Scholar] [CrossRef]
- Evans, A.; Whelehan, P.; Thomson, K.; McLean, D.; Brauer, K.; Purdie, C.; Jordan, L.; Baker, L.; Thompson, A. Quantitative shear wave ultrasound elastography: Initial experience in solid breast masses. Breast Cancer Res. 2010, 12, R104. [Google Scholar] [CrossRef] [Green Version]
- Denis, M.; Mehrmohammadi, M.; Song, P.; Meixner, D.D.; Fazzio, R.T.; Pruthi, S.; Whaley, D.H.; Chen, S.; Fatemi, M.; Alizad, A. Comb-push ultrasound shear elastography of breast masses: Initial results show promise. PLoS ONE 2015, 10, e0119398. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Odulate, A.; Ong, E.M.; Chikarmane, S.; Harston, C.W. Using real-time tissue elastography for breast lesion evaluation: Our initial experience. J. Ultrasound Med. 2010, 29, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.H.; Jung, H.K.; Lee, J.T.; Ko, K.H. Shear-wave elastography in the diagnosis of solid breast masses: What leads to false-negative or false-positive results? Eur. Radiol. 2013, 23, 2432–2440. [Google Scholar] [CrossRef]
- Youk, J.H.; Gweon, H.M.; Son, E.J.; Han, K.H.; Kim, J.A. Diagnostic value of commercially available shear-wave elastography for breast cancers: Integration into BI-RADS classification with subcategories of category 4. Eur. Radiol. 2013, 23, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.; Mehrmohammadi, M.; Denis, M.; Bayat, M.; Stan, D.L.; Fatemi, M.; Alizad, A. Effect of calcifications on breast ultrasound shear wave elastography: An investigational study. PLoS ONE 2015, 10, e0137898. [Google Scholar] [CrossRef] [PubMed]
- Madani, N.; Mojra, A. Quantitative diagnosis of breast tumors by characterization of viscoelastic behavior of healthy breast tissue. J. Mech. Behav. Biomed. Mater. 2017, 68, 180–187. [Google Scholar] [CrossRef]
- Qiu, Y.; Sridhar, M.; Tsou, J.K.; Lindfors, K.K.; Insana, M.F. Ultrasonic viscoelasticity imaging of nonpalpable breast tumors: Preliminary results. Acad. Radiol. 2008, 15, 1526–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayat, M.; Nabavizadeh, A.; Kumar, V.; Gregory, A.; Insana, M.; Alizad, A.; Fatemi, M. AutomatedIn VivoSub-Hertz Analysis of Viscoelasticity (SAVE) for Evaluation of Breast Lesions. IEEE Trans. Biomed. Eng. 2017, 65, 2237–2247. [Google Scholar] [CrossRef]
- Amy, D.; Bercoff, J.; Bibby, E. Breast Elastography. In Lobar Approach to Breast Ultrasound; Springer: New York, NY, USA, 2018; pp. 85–106. [Google Scholar]
- Barr, R.G. Future of breast elastography. Ultrasonography 2019, 38, 93. [Google Scholar] [CrossRef] [Green Version]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2018, 70, 151–171. [Google Scholar] [CrossRef]
- Byass, P. The global burden of liver disease: A challenge for methods and for public health. BMC Med. 2014, 12, 159. [Google Scholar] [CrossRef] [Green Version]
- Marcellin, P.; Kutala, B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Ferraioli, G.; Wong, V.W.S.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver ultrasound elastography: An update to the world federation for ultrasound in medicine and biology guidelines and recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef] [Green Version]
- Ferraioli, G.; Filice, C.; Castera, L.; Choi, B.I.; Sporea, I.; Wilson, S.R.; Cosgrove, D.; Dietrich, C.F.; Amy, D.; Bamber, J.C.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: Liver. Ultrasound Med. Biol. 2015, 41, 1161–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, R.G.; Ferraioli, G.; Palmeri, M.L.; Goodman, Z.D.; Garcia-Tsao, G.; Rubin, J.; Garra, B.; Myers, R.P.; Wilson, S.R.; Rubens, D.; et al. Elastography assessment of liver fibrosis: Society of radiologists in ultrasound consensus conference statement. Radiology 2015, 276, 845–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for Study of Liver. EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J. Hepatol. 2014, 60, 392. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version). Ultraschall Med. Eur. J. Ultrasound 2017, 38, e16–e47. [Google Scholar]
- Gebo, K.A.; Herlong, H.F.; Torbenson, M.S.; Jenckes, M.W.; Chander, G.; Ghanem, K.G.; El-Kamary, S.S.; Sulkowski, M.; Bass, E.B. Role of liver biopsy in management of chronic hepatitis C: A systematic review. Hepatology 2002, 36, s161–s172. [Google Scholar] [CrossRef] [Green Version]
- Seeff, L.B.; Everson, G.T.; Morgan, T.R.; Curto, T.M.; Lee, W.M.; Ghany, M.G.; Shiffman, M.L.; Fontana, R.J.; Di Bisceglie, A.M.; Bonkovsky, H.L.; et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin. Gastroenterol. Hepatol. 2010, 8, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Regev, A.; Berho, M.; Jeffers, L.J.; Milikowski, C.; Molina, E.G.; Pyrsopoulos, N.T.; Feng, Z.Z.; Reddy, K.R.; Schiff, E.R. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am. J. Gastroenterol. 2002, 97, 2614. [Google Scholar] [CrossRef]
- Stotland, B.; Lichtenstein, G. Liver biopsy complications and routine ultrasound. Am. J. Gastroenterol. 1996, 91, 1295. [Google Scholar]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef]
- Ziol, M.; Handra-Luca, A.; Kettaneh, A.; Christidis, C.; Mal, F.; Kazemi, F.; de Lédinghen, V.; Marcellin, P.; Dhumeaux, D.; Trinchet, J.C.; et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005, 41, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Castéra, L.; Vergniol, J.; Foucher, J.; Le Bail, B.; Chanteloup, E.; Haaser, M.; Darriet, M.; Couzigou, P.; de Lédinghen, V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005, 128, 343–350. [Google Scholar] [CrossRef]
- Chon, Y.E.; Choi, E.H.; Song, K.J.; Park, J.Y.; Han, K.H.; Chon, C.Y.; Ahn, S.H.; Kim, S.U. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: A meta-analysis. PLoS ONE 2012, 7, e44930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afdhal, N.H.; Bacon, B.R.; Patel, K.; Lawitz, E.J.; Gordon, S.C.; Nelson, D.R.; Challies, T.L.; Nasser, I.; Garg, J.; Wei, L.J.; et al. Accuracy of fibroscan, compared with histology, in analysis of liver fibrosis in patients with hepatitis B or C: A United States multicenter study. Clin. Gastroenterol. Hepatol. 2015, 13, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Cassinotto, C.; Lapuyade, B.; Mouries, A.; Hiriart, J.B.; Vergniol, J.; Gaye, D.; Castain, C.; Le Bail, B.; Chermak, F.; Foucher, J.; et al. Non-invasive assessment of liver fibrosis with impulse elastography: Comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J. Hepatol. 2014, 61, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Isselin, G.; Fouchard-Hubert, I.; Oberti, F.; Dib, N.; Lebigot, J.; Bertrais, S.; Gallois, Y.; Calès, P.; Aubé, C. Acoustic radiation force impulse: A new ultrasonographic technology for the widespread noninvasive diagnosis of liver fibrosis. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1074–1084. [Google Scholar] [CrossRef] [Green Version]
- Palmeri, M.L.; Wang, M.H.; Dahl, J.J.; Frinkley, K.D.; Nightingale, K.R. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med. Biol. 2008, 34, 546–558. [Google Scholar] [CrossRef] [Green Version]
- Muller, M.; Gennisson, J.L.; Deffieux, T.; Tanter, M.; Fink, M. Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: Preliminary in vivo feasability study. Ultrasound Med. Biol. 2009, 35, 219–229. [Google Scholar] [CrossRef]
- Deffieux, T.; Montaldo, G.; Tanter, M.; Fink, M. Shear wave spectroscopy for in vivo quantification of human soft tissues visco-elasticity. IEEE Trans. Med. Imaging 2008, 28, 313–322. [Google Scholar] [CrossRef]
- Barry, C.T.; Hah, Z.; Partin, A.; Mooney, R.A.; Chuang, K.H.; Augustine, A.; Almudevar, A.; Cao, W.; Rubens, D.J.; Parker, K.J. Mouse liver dispersion for the diagnosis of early-stage fatty liver disease: A 70-sample study. Ultrasound Med. Biol. 2014, 40, 704–713. [Google Scholar] [CrossRef]
- Huwart, L.; Sempoux, C.; Salameh, N.; Jamart, J.; Annet, L.; Sinkus, R.; Peeters, F.; ter Beek, L.C.; Horsmans, Y.; Van Beers, B.E. Liver fibrosis: Noninvasive assessment with MR elastography versus aspartate aminotransferase–to-platelet ratio index. Radiology 2007, 245, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Millonig, G.; Friedrich, S.; Adolf, S.; Fonouni, H.; Golriz, M.; Mehrabi, A.; Stiefel, P.; Pöschl, G.; Büchler, M.W.; Seitz, H.K.; et al. Liver stiffness is directly influenced by central venous pressure. J. Hepatol. 2010, 52, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Bavu, É.; Gennisson, J.L.; Couade, M.; Bercoff, J.; Mallet, V.; Fink, M.; Badel, A.; Vallet-Pichard, A.; Nalpas, B.; Tanter, M.; et al. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: A clinical study on 113 hepatitis C virus patients. Ultrasound Med. Biol. 2011, 37, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Sporea, I.; Bota, S.; Gradinaru-Taşcău, O.; Şirli, R.; Popescu, A.; Jurchiş, A. Which are the cut-off values of 2D-Shear Wave Elastography (2D-SWE) liver stiffness measurements predicting different stages of liver fibrosis, considering Transient Elastography (TE) as the reference method? Eur. J. Radiol. 2014, 83, e118–e122. [Google Scholar] [CrossRef]
- Benedict, M.; Zhang, X. Non-alcoholic fatty liver disease: An expanded review. World J. Hepatol. 2017, 9, 715. [Google Scholar] [CrossRef]
- Sherman, K.E.; Abdel-Hameed, E.A.; Ehman, R.L.; Rouster, S.D.; Campa, A.; Martinez, S.S.; Huang, Y.; Zarini, G.G.; Hernandez, J.; Teeman, C.; et al. Validation and refinement of noninvasive methods to assess hepatic fibrosis: Magnetic resonance elastography versus enhanced liver fibrosis index. Dig. Dis. Sci. 2019, 65, 1252–1257. [Google Scholar] [CrossRef]
- Ajmera, V.; Liu, A.; Singh, S.; Yachoa, G.; Ramey, M.; Bhargava, M.; Zamani, A.; Lopez, S.; Mangla, N.; Bettencourt, R.; et al. Clinical Utility of an Increase in Magnetic Resonance Elastography in Predicting Fibrosis Progression in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 849–860. [Google Scholar] [CrossRef]
- Kang, K.A.; Jun, D.W.; Kim, M.S.; Kwon, H.J.; Nguyen, M.H. Prevalence of significant hepatic fibrosis using magnetic resonance elastography in a health check-up clinic population. Aliment. Pharmacol. Ther. 2020, 51, 388–396. [Google Scholar] [CrossRef]
- O’Hara, S.; Zelesco, M.; Sun, Z. Can shear wave elastography of the cervix be of use in predicting imminent cervical insufficiency and preterm birth?—Preliminary results. Ultrasound Med. Biol. 2019, 45, S111–S112. [Google Scholar] [CrossRef]
- Thomas, A. Imaging of the cervix using sonoelastography. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2006, 28, 356–357. [Google Scholar] [CrossRef]
- Feltovich, H.; Hall, T. Quantitative imaging of the cervix: Setting the bar. Ultrasound Obstet. Gynecol. 2013, 41, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Badir, S.; Pensalfini, M.; Bajka, M.; Abitabile, P.; Zimmermann, R.; Mazza, E. Challenging the in-vivo assessment of biomechanical properties of the uterine cervix: A critical analysis of ultrasound based quasi-static procedures. J. Biomech. 2015, 48, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Molina, F.; Gómez, L.; Florido, J.; Padilla, M.; Nicolaides, K. Quantification of cervical elastography: A reproducibility study. Ultrasound Obstet. Gynecol. 2012, 39, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Andrade, E.; Hassan, S.S.; Ahn, H.; Korzeniewski, S.J.; Yeo, L.; Chaiworapongsa, T.; Romero, R. Evaluation of cervical stiffness during pregnancy using semiquantitative ultrasound elastography. Ultrasound Obstet. Gynecol. 2013, 41, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hee, L.; Sandager, P.; Petersen, O.; Uldbjerg, N. Quantitative sonoelastography of the uterine cervix by interposition of a synthetic reference material. Acta Obstet. Gynecol. Scand. 2013, 92, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Mazza, E.; Parra-Saavedra, M.; Bajka, M.; Gratacos, E.; Nicolaides, K.; Deprest, J. In vivo assessment of the biomechanical properties of the uterine cervix in pregnancy. Prenat. Diagn. 2014, 34, 33–41. [Google Scholar] [CrossRef]
- Fruscalzo, A.; Mazza, E.; Feltovich, H.; Schmitz, R. Cervical elastography during pregnancy: A critical review of current approaches with a focus on controversies and limitations. J. Med. Ultrason. 2016, 43, 493–504. [Google Scholar] [CrossRef]
- Nightingale, K.; Palmeri, M.; Trahey, G. Analysis of contrast in images generated with transient acoustic radiation force. Ultrasound Med. Biol. 2006, 32, 61–72. [Google Scholar] [CrossRef]
- Carlson, L.C.; Romero, S.T.; Palmeri, M.L.; del Rio, A.M.; Esplin, S.M.; Rotemberg, V.M.; Hall, T.; Feltovich, H.M. Changes in shear wave speed pre- and post-induction of labor: A feasibility study. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2015, 46, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Muller, M.; Aït-Belkacem, D.; Hessabi, M.; luc Gennisson, J.; Grange, G.; Goffinet, F.; Lecarpentier, E.R.; Cabrol, D.; Tanter, M.; Tsatsaris, V. Assessment of the Cervix in Pregnant Women Using Shear Wave Elastography: A Feasibility Study. Ultrasound Med. Biol. 2015, 41, 2789–2797. [Google Scholar] [CrossRef]
- Hernández-Andrade, E.; Aurioles-Garibay, A.; Garcia, M.; Korzeniewski, S.J.; Schwartz, A.G.; Ahn, H.; Martínez-Varea, A.; Yeo, L.; Chaiworapongsa, T.; Hassan, S.S.; et al. Effect of depth on shear-wave elastography estimated in the internal and external cervical os during pregnancy. J. Perinat. Med. 2014, 42, 549–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, L.C.; Hall, T.J.; Rosado-Mendez, I.M.; Palmeri, M.L.; Feltovich, H.M. Detection of Changes in Cervical Softness Using Shear Wave Speed in Early versus Late Pregnancy: An in Vivo Cross-Sectional Study. Ultrasound Med. Biol. 2017, 44, 515–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosado-Mendez, I.M.; Carlson, L.C.; Woo, K.M.; Santoso, A.P.; Guerrero, Q.W.; Palmeri, M.L.; Feltovich, H.; Hall, T.J. Quantitative assessment of cervical softening during pregnancy in the Rhesus macaque with shear wave elasticity imaging. Phys. Med. Biol. 2018, 63, 085016. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.C.; Hall, T.J.; Rosado-Mendez, I.M.; Mao, L.; Feltovich, H. Quantitative assessment of cervical softening during pregnancy with shear wave elasticity imaging: An in vivo longitudinal study. Interface Focus 2019, 9, 20190030. [Google Scholar] [CrossRef] [Green Version]
- Peralta, L.; Molina, F.; Melchor, J.; Gomez, L.; Masso, P.; Florido, J.; Rus, G. Transient Elastography to Assess the Cervical Ripening during Pregnancy: A Preliminary Study. Ultrasound Obstet. Gynecol. 2015, 38, 395–402. [Google Scholar] [CrossRef]
- Hernández-Andrade, E.; Romero, R.; Korzeniewski, S.J.; Ahn, H.; Aurioles-Garibay, A.; Garcia, M.; Schwartz, A.G.; Yeo, L.; Chaiworapongsa, T.; Hassan, S.S. Cervical strain determined by ultrasound elastography and its association with spontaneous preterm delivery. J. Perinat. Med. 2014, 42, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Reissner, E.; Sagoci, H. Forced torsional oscillations of an elastic half-space. I. J. Appl. Phys. 1944, 15, 652–654. [Google Scholar] [CrossRef]
- Hadj Henni, A.; Schmitt, C.; Trop, I.; Cloutier, G. Shear wave induced resonance elastography of spherical masses with polarized torsional waves. Appl. Phys. Lett. 2012, 100, 133702. [Google Scholar] [CrossRef] [Green Version]
- Ouared, A.; Montagnon, E.; Cloutier, G. Generation of remote adaptive torsional shear waves with an octagonal phased array to enhance displacements and reduce variability of shear wave speeds: Comparison with quasi-plane shear wavefronts. Phys. Med. Biol. 2015, 60, 8161. [Google Scholar] [CrossRef]
- Yengul, S.S.; Barbone, P.E.; Madore, B. Dispersion in Tissue-Mimicking Gels Measured with Shear Wave Elastography and Torsional Vibration Rheometry. Ultrasound Med. Biol. 2019, 45, 586–604. [Google Scholar] [CrossRef]
- Melchor, J.; Rus, G. Torsional ultrasonic transducer computational design optimization. Ultrasonics 2014, 54, 1950–1962. [Google Scholar] [CrossRef] [PubMed]
- Massó, P.; Callejas, A.; Melchor, J.; Molina, F.S.; Rus, G. In Vivo Measurement of Cervical Elasticity on Pregnant Women by Torsional Wave Technique: A Preliminary Study. Sensors 2019, 19, 3249. [Google Scholar] [CrossRef] [Green Version]
- McFarlin, B.L.; Kumar, V.; Bigelow, T.A.; Simpson, D.G.; White-Traut, R.C.; Abramowicz, J.S.; O’Brien, W.D. Beyond Cervical Length: A Pilot Study of Ultrasonic Attenuation for Early Detection of Preterm Birth Risk. Ultrasound Med. Biol. 2015, 41, 3023–3029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, K.M.; Socrate, S.; Paskaleva, A.; House, M. A study of the anisotropy and tension/compression behavior of human cervical tissue. J. Biomech. Eng. 2010, 132, 021003. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Asbach, P.; Streitberger, K.; Thomas, A.Z.; Hamm, B.; Braun, J.; Sack, I.; Guo, J. In vivo high-resolution magnetic resonance elastography of the uterine corpus and cervix. Eur. Radiol. 2014, 24, 3025–3033. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yao, W.; Gan, Y.; Zhao, L.; McKee, W.E.; Vink, J.; Wapner, R.; Hendon, C.; Myers, K. Anisotropic Material Characterization of Human Cervix Tissue based on Indentation. J. Biomech. Eng. 2019, 141, 091017. [Google Scholar] [CrossRef]






| Technique | Soft Tissue | Study Objective | Method | Reference |
|---|---|---|---|---|
| SDUV | Liver in vivo porcine | Regular characterization | Dispersion curve Voigt model | Chen et al. [98] |
| Liver in vivo | Regular characterization | Dispersion curve Voigt model | Chen et al. [99] | |
| Liver in vitro rat | Fibrosis staging | Dispersion curve Voigt model | Lin et al. [100] | |
| Prostate in vitro | Regular characterization | Dispersion curve Voigt model | Mitri et al. [101] | |
| Breast in vivo | Malignant vs. Benign vs. Healthy state | Dispersion curve Voigt model | Kumar et al. [89] | |
| DMA | Prostate in vitro | Healthy vs. Cancerous state | Dispersion curve KVFD model | Zhang et al. [102] |
| MRE | Breast in vivo | Malignant vs. Benign vs. Healthy state | Phase offset imaging reconstruction | Sinkus et al. [103] |
| Breast in vivo | Malignant vs. Benign vs. Healthy state | Transversely isotropic model | Sinkus et al. [104] | |
| Liver in vivo | Transplant rejection | Attenuation Measuring Ultrasound Shearwave Elastography (AMUSE) | Nenadic et al. [105] | |
| Liver in vivo | Regular characterization | Dispersion curve Zener model | Klatt et al. [106] | |
| Liver in vivo | Fibrosis staging | Dispersion curve Zener model | Asbach et al. [107] | |
| Prostate in vivo | Prostate cancer vs. Benign prostatitis | Phase offset imaging reconstruction | Li et al. [108] | |
| SWE | Liver in vivo | Fibrosis | Shear Wave Dispersion Slope | Sugimoto et al. [109] |
| Liver in vivo | Healthy vs. Fibrosis staging | Shear Wave Spectroscopy | Deffieux et al. [110] | |
| TWE | Cervix Ex vivo | Regular characterization | Dispersion curve KV and KVFD model | Callejas et al. [111] |
| Method | Advantages | Disadvantages |
|---|---|---|
| Shear wave speed dispersion curve: estimation of vicosity parameters by fitting a rheological model | Most relevant and extended technique Considerable amount of previous work for different types of organs to compare with Depends on shear wave methods: noninvasive both internally and externally in contact with the soft tissue | No consensus on the most appropriate rheological model for soft tissue characterization Studies report values of viscosity for a specific rheological model (not comparable) |
| Shear Wave Dispersion Imaging | Dispersion slope value: physical quantity not based on a rheological model (model-free) | Integrated into commercial ultrasound systems not accessible for researchers (black box software) |
| Shear Wave Spectroscopy: new signal processing of the SSI data (Supersonic Shear Imaging) | Frequency-dependent measurement of the shear wave speed, quantitative and noninvasive | Limits its use to scans via SSI |
| Advantages | Disadvantages |
|---|---|
| New set of parameters to interpret biological and physiological disorders | Several proposed models to be chosen depending on the problem, pathology or tissue considered |
| Characterization of tissue microscale in terms of harmonics | Inhomogeneus measurements due to the nature of propagation in the tissue |
| Open questions that add a new branch in biomedical engineering | Mathematically intractable in exact terms |
| Tissue State | Viscosity Parameter (Pa.s) | Fractional Derivate Order | Method | Reference |
|---|---|---|---|---|
| Healthy | 3.61 ± 1.25 | 0.215 ± 0.042 | DMA | Zhang et al. [102] |
| Cancerous | 8.65 ± 3.40 | 0.225 ± 0.03 | ||
| Healthy | 1.10–6.82 (range) | - | SDUV | Mitri et al. [101] |
| Benign prostatitis | 2.13 ± 0.21 | - | MRE | Li et al. [108] |
| Cancerous | 6.56 ± 0.99 | - |
| Tissue State | Viscosity Parameter (Pa.s) | Method | Reference |
|---|---|---|---|
| Malignant | 2.40 ± 1.70 | MRE | Sinkus et al. [103] |
| Benign | 2.10 ± 1.40 | ||
| Healthy | 0.55 ± 0.12 | ||
| Malignant | 3.00 ± 0.80 | Transverse Acoustic Waves | Sinkus et al. [104] |
| Benign | 2.40 ± 1.90 | ||
| Healthy | 0.70 ± 0.55 | ||
| Malignant | 8.22 ± 3.36 | SDUV + Kelvin-Voigt | Kumar et al. [89] |
| Benign | 2.83 ± 1.47 | ||
| Healthy | 1.41 ± 0.67 |
| Tissue State | Viscosity Parameter (Pa.s) | Method | Reference |
|---|---|---|---|
| Healthy | 6.7 ± 1.3 | MRE + Zener model | Klatt et al. [106] |
| Healthy | 7.3 ± 2.3 | MRE + Zener model | Asbach et al. [107] |
| Healthy | 2.0 ± 0.8 (F0) | SW spectroscopy | Deffieux et al. [110] |
| 2.3 ± 0.7 (F1) | |||
| Fibrosis | 2.6 ± 0.5 (F2) | SW spectroscopy | Deffieux et al [110] |
| 2.7 ± 1.9 (F3) | |||
| 3.7 ± 2.5 (F4) | |||
| Fibrosis | 14.4 ± 6.6 (F3–4) | MRE + Zener model | Asbach et al. [107] |
| Models | Rheometry (R) | TWE | R + TWE |
|---|---|---|---|
| Elasticity (kPa) | |||
| KV | 1.79 ± 0.08 | 2.43 ± 0.26 | 1.92 ± 0.15 |
| KVFD | 0.92 ± 0.15 | 2.06 ± 0.11 | 2.01 ± 0.24 |
| Viscosity (Pa.s) | |||
| KV | 6.34 ± 0.95 | 4.59 ± 0.29 | 4.5 ± 0.25 |
| KVFD | 23 ± 9.84 | 4.23 ± 0.22 | 4.64 ± 0.09 |
| Fractional Derivative Power | |||
| KVFD | 0.25 ± 0.15 | 0.97 ± 0.02 | 0.98 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rus, G.; Faris, I.H.; Torres, J.; Callejas, A.; Melchor, J. Why Are Viscosity and Nonlinearity Bound to Make an Impact in Clinical Elastographic Diagnosis? Sensors 2020, 20, 2379. https://doi.org/10.3390/s20082379
Rus G, Faris IH, Torres J, Callejas A, Melchor J. Why Are Viscosity and Nonlinearity Bound to Make an Impact in Clinical Elastographic Diagnosis? Sensors. 2020; 20(8):2379. https://doi.org/10.3390/s20082379
Chicago/Turabian StyleRus, Guillermo, Inas H. Faris, Jorge Torres, Antonio Callejas, and Juan Melchor. 2020. "Why Are Viscosity and Nonlinearity Bound to Make an Impact in Clinical Elastographic Diagnosis?" Sensors 20, no. 8: 2379. https://doi.org/10.3390/s20082379
APA StyleRus, G., Faris, I. H., Torres, J., Callejas, A., & Melchor, J. (2020). Why Are Viscosity and Nonlinearity Bound to Make an Impact in Clinical Elastographic Diagnosis? Sensors, 20(8), 2379. https://doi.org/10.3390/s20082379