Analytical Parameters of a Novel Glucose Biosensor Based on Grafted PFM as a Covalent Immobilization Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
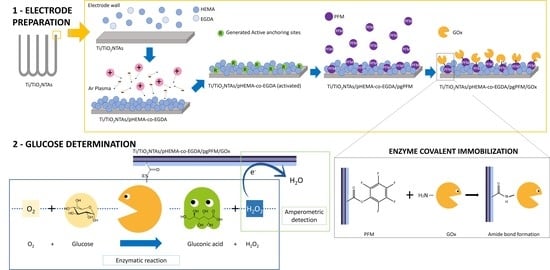
2.2. Biosensor Preparation
2.3. Electrochemical Measurements
2.4. Glucose Determination in Food Samples
3. Results and Discussion
3.1. Evaluation of the Biosensor Enzymatic Activity
3.2. Evaluation of Accuracy
3.3. Evaluation of Precision
3.4. Evaluation of Robustness
3.5. Evaluation of Long-Term Stability
3.6. Food Sample Quantification
- Electrochemical interfaces based on titanium and titanium (IV) oxide promote the biocompatibility of the system; titanium is a pharmacologically inert metal that does not cause allergic reactions in the immune system and the human body does not reject it. Moreover, TiO2NTAs offers a high surface area and the ability to promote charge transfer processes. Thus, Ti/TiO2NTAs is an excellent electrochemical interface for implantable biosensors.
- Chitosan acts as a protective barrier for the immobilized enzyme and provides conformational stability to the measurement device. Chitosan is a biocompatible hydrogel obtained from natural sources that prevents enzyme denaturalization because of its high affinity for proteins [60]. Moreover, chitosan preserves its structure under adverse conditions due to its high mechanical stability. For this reason, using chitosan, the long-term stability of the biosensors is improved. The use of chitosan also improves the biosensor’s sensitivity because this polymeric matrix blocks possible interfering macromolecules.
- Poly-HEMA protects the electrochemical interface from adhesion of molecules that can interfere with the analytical measurement and/or cause damage to the human body, generating a foreign body response [61]. Therefore, the risk of rejection of implantable devices is reduced by using this polymer in the biosensor’ architecture. Furthermore, by using plasma techniques for HEMA polymerization, the obtained films follow the shape of the electrochemical interface [62]. Thus, ppHEMA-co-EGDA films follow the architecture of the TiO2NTAs, and both high specific surface area and high sensitivity are maintained.
- Covalent immobilization of enzyme molecules provides several benefits. First, it decreases the probability of enzyme leakage from the biosensor architecture. Second, covalent configuration ensures that enzymes are located close enough to the transducer to avoid the loss of the electrons produced from the enzymatic reaction. In addition, the pgPFM surface shows high covalency, which is the ability of the surface to retain the attached molecules after vigorous washing [63]. Hence, the developed biosensors are likely to retain their sensitivity under milder conditions, such as those found in the human bloodstream. Finally, the PFM plasma-grafting modification minimizes enzyme deactivation during the immobilization process [38].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical Affinity Biosensors Based on Selected Nanostructures for Food and Environmental Monitoring. Sensors 2020, 20, 5125. [Google Scholar] [CrossRef]
- McConnell, E.M.; Nguyen, J.; Li, Y. Aptamer-Based Biosensors for Environmental Monitoring. Front. Chem. 2020, 8, 434. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Wang, H.; He, D.; Wan, K.; Sheng, X.; Cheng, H.; Huang, J.; Zhou, X.; He, X.; Wang, K. In situ multiplex detection of serum exosomal microRNAs using an all-in-one biosensor for breast cancer diagnosis. Analyst 2020, 145, 3289–3296. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Wu, S.; Pan, Y.; Dong, Y.; Zhu, S.; Yang, J.; Yin, Y.; Li, G. An Electrochemical Biosensor Designed by Using Zr-Based Metal–Organic Frameworks for the Detection of Glioblastoma-Derived Exosomes with Practical Application. Anal. Chem. 2020, 92, 3819–3826. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Tong, L.; Shen, Y.; Zhu, W.; Yin, L.; Huang, S.; Zhu, F.; Chen, G.; Ouyang, G. Smartphone-assisted robust enzymes@MOFs-based paper biosensor for point-of-care detection. Biosens. Bioelectron. 2020, 156, 112095. [Google Scholar] [CrossRef] [PubMed]
- Bougadi, E.T.; Kalogianni, D.P. Paper-based DNA biosensor for food authenticity testing. Food Chem. 2020, 322, 126758. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ji, J.; Sun, Z.; Shen, P.; Sun, X. Recent advances in electrochemical biosensors for antioxidant analysis in foodstuff. TrAC Trends Anal. Chem. 2020, 122, 115718. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. TrAC Trends Anal. Chem. 2020, 124, 115814. [Google Scholar] [CrossRef]
- Bettazzi, F.; Marrazza, G.; Minunni, M.; Palchetti, I.; Scarano, S. Biosensors and Related Bioanalytical Tools. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–33. [Google Scholar]
- Lee, H.-B.; Meeseepong, M.; Trung, T.Q.; Kim, B.-Y.; Lee, N.-E. A wearable lab-on-a-patch platform with stretchable nanostructured biosensor for non-invasive immunodetection of biomarker in sweat. Biosens. Bioelectron. 2020, 156, 112133. [Google Scholar] [CrossRef]
- Su, Y.; Yang, T.; Zhao, X.; Cai, Z.; Chen, G.; Yao, M.; Chen, K.; Bick, M.; Wang, J.; Li, S.; et al. A wireless energy transmission enabled wearable active acetone biosensor for non-invasive prediabetes diagnosis. Nano Energy 2020, 74, 104941. [Google Scholar] [CrossRef]
- Karpova, E.V.; Karyakina, E.E.; Karyakin, A.A. Wearable non-invasive monitors of diabetes and hypoxia through continuous analysis of sweat. Talanta 2020, 215, 120922. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, W.; Xu, T.; Chen, H.; Jin, Z.; Zhang, Z.; Song, Q.; Tang, Y. Miniaturized Paper-Based Smartphone Biosensor for Differential Diagnosis of Wild-type Pseudorabies Virus Infection versus Vaccination Immunization. Sens. Actuators B Chem. 2021, 327, 128893. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Yang, C.; Ma, C.; Tang, J. A smartphone-assisted portable biosensor using laccase-mineral hybrid microflowers for colorimetric determination of epinephrine. Talanta 2021, 224, 121840. [Google Scholar] [CrossRef]
- Otero, F.; Magner, E. Biosensors—Recent Advances and Future Challenges in Electrode Materials. Sensors 2020, 20, 3561. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Schoenitz, M.; Grundemann, L.; Augustin, W.; Scholl, S. Fouling in microstructured devices: A review. Chem. Commun. 2015, 51, 8213–8228. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lee, H. Anti-Biofouling Strategies for Long-Term Continuous Use of Implantable Biosensors. Chemosensors 2020, 8, 66. [Google Scholar] [CrossRef]
- Abraham, A.A.; Means, A.K.; Clubb, F.J.; Fei, R.; Locke, A.K.; Gacasan, E.G.; Coté, G.L.; Grunlan, M.A. Foreign Body Reaction to a Subcutaneously Implanted Self-Cleaning, Thermoresponsive Hydrogel Membrane for Glucose Biosensors. ACS Biomater. Sci. Eng. 2018, 4, 4104–4111. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, H.; Liu, W.; Wang, Y.; Pang, W.; Duan, X. Biofouling Removal and Protein Detection Using a Hypersonic Resonator. ACS Sens. 2017, 2, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Raffiee, A.H.; John, S.W.M.; Ardekani, A.M.; Lee, H. Towards smart self-clearing glaucoma drainage device. Microsyst. Nanoeng. 2018, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, M.; Jiao, M.; Luo, X. Antifouling and ultrasensitive biosensing interface based on self-assembled peptide and aptamer on macroporous gold for electrochemical detection of immunoglobulin E in serum. Anal. Bioanal. Chem. 2018, 410, 5871–5878. [Google Scholar] [CrossRef]
- Yu, B.; Wang, C.; Ju, Y.M.; West, L.; Harmon, J.; Moussy, Y.; Moussy, F. Use of hydrogel coating to improve the performance of implanted glucose sensors. Biosens. Bioelectron. 2008, 23, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lee, C.-J.; Wang, H.; Hu, Y.; Young, M.; Han, Y.; Xu, F.-J.; Cong, H.; Cheng, G. Highly sensitive and stable zwitterionic poly(sulfobetaine-3,4-ethylenedioxythiophene) (PSBEDOT) glucose biosensor. Chem. Sci. 2018, 9, 2540–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Miao, J.; Yan, J.; Yang, K.; Mao, C.; Ju, J.; Shen, J. Applications of antibiofouling PEG-coating in electrochemical biosensors for determination of glucose in whole blood. Electrochim. Acta 2013, 89, 549–554. [Google Scholar] [CrossRef]
- Chan, D.; Chien, J.-C.; Axpe, E.; Blankemeier, L.; Baker, S.; Swaminathan, S.; Piunova, V.; Zubarev, D.Y.; Maikawa, C.; Soh, H.T.; et al. Combinatorial Polyacrylamide Hydrogels for Preventing Biofouling on Implantable Biosensors. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tanaka, M.; Mochizuki, A.; Ishii, N.; Motomura, T.; Hatakeyama, T. Study of blood compatibility with poly(2-methoxyethyl acrylate). Relationship between water structure and platelet compatibility in poly(2-methoxyethylacrylate-co-2-hydroxyethylmethacrylate). Biomacromolecules 2002, 3, 36–41. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ishihara, K. Phosphorylcholine-containing polymers for biomedical applications. Anal. Bioanal. Chem. 2005, 381, 534–546. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C. Surface field of forces and protein adsorption behavior of poly(hydroxyethylmethacrylate) films deposited from plasma. J. Biomed. Mater. Res. 1995, 29, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Malone-Povolny, M.J.; Merricks, E.P.; Wimsey, L.E.; Nichols, T.C.; Schoenfisch, M.H. Long-Term Accurate Continuous Glucose Biosensors via Extended Nitric Oxide Release. ACS Sens. 2019, 4, 3257–3264. [Google Scholar] [CrossRef]
- Li, P.; Lee, G.-H.; Kim, S.Y.; Kwon, S.Y.; Kim, H.-R.; Park, S. From Diagnosis to Treatment: Recent Advances in Patient-Friendly Biosensors and Implantable Devices. ACS Nano 2021, 15, 1960–2004. [Google Scholar] [CrossRef]
- Li, H.; Dauphin-Ducharme, P.; Ortega, G.; Plaxco, K.W. Calibration-Free Electrochemical Biosensors Supporting Accurate Molecular Measurements Directly in Undiluted Whole Blood. J. Am. Chem. Soc. 2017, 139, 11207–11213. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-T.; Clark, K.; Ma, W.; Mulchandani, A. Detection of a secreted protein biomarker for citrus Huanglongbing using a single-walled carbon nanotubes-based chemiresistive biosensor. Biosens. Bioelectron. 2020, 147, 111766. [Google Scholar] [CrossRef]
- Muñoz-San Martín, C.; Pedrero, M.; Gamella, M.; Montero-Calle, A.; Barderas, R.; Campuzano, S.; Pingarrón, J.M. A novel peptide-based electrochemical biosensor for the determination of a metastasis-linked protease in pancreatic cancer cells. Anal. Bioanal. Chem. 2020, 412, 6177–6188. [Google Scholar] [CrossRef]
- Bilgi, M.; Ayranci, E. Development of amperometric biosensors using screen-printed carbon electrodes modified with conducting polymer and nanomaterials for the analysis of ethanol, methanol and their mixtures. J. Electroanal. Chem. 2018, 823, 588–592. [Google Scholar] [CrossRef]
- Artigues, M.; Abellà, J.; Colominas, S. Analytical Parameters of an Amperometric Glucose Biosensor for Fast Analysis in Food Samples. Sensors 2017, 17, 2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artigues, M.; Oh, S.; Gilabert-Porres, J.; Abellà, J.; Borrós, S.; Colominas, S. Novel grafted electrochemical interface for covalent glucose oxidase immobilization using reactive pentafluorophenyl methacrylate. Colloids Surf. B Biointerfaces 2019, 175. [Google Scholar] [CrossRef] [PubMed]
- AOAC Official Method. AOAC 977.20-1977, Separation of Sugars in Honey, Liquid Chromatographic Method. JAOAC 1977, 60, 838. [Google Scholar]
- Francesch, L.; Borros, S.; Knoll, W.; Förch, R. Surface reactivity of pulsed-plasma polymerized pentafluorophenyl methacrylate (PFM) toward amines and proteins in solution. Langmuir 2007, 23, 3927–3931. [Google Scholar] [CrossRef]
- Duque, L.; Queralto, N.; Francesch, L.; Bumbu, G.G.; Borros, S.; Berger, R.; Förch, R. Reactions of plasma-polymerised pentafluorophenyl methacrylate with simple amines. Plasma Process. Polym. 2010, 7, 915–925. [Google Scholar] [CrossRef]
- Duque, L.; Menges, B.; Borros, S.; Förch, R. Immobilization of biomolecules to plasma polymerized pentafluorophenyl methacrylate. Biomacromolecules 2010, 11, 2818–2823. [Google Scholar] [CrossRef]
- Montero, L.; Baxamusa, S.H.; Borros, S.; Gleason, K.K. Thin hydrogel films with nanoconfined surface reactivity by photoinitiated chemical vapor deposition. Chem. Mater. 2009, 21, 399–403. [Google Scholar] [CrossRef]
- Queralto, N.; Bumbu, G.G.; Francesch, L.; Knoll, W.; Borros, S.; Berger, R.; Förch, R. Investigation into the Chemical Reactivity of Plasma-Deposited Perfluorophenyl Methacrylate Using Infrared Reflection Absorption Spectroscopy and Microcantilever Studies. Plasma Process. Polym. 2007, 4, S790–S793. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Cheng, Y.; Jiang, S.P. Effect of Carbon Nanotubes on Direct Electron Transfer and Electrocatalytic Activity of Immobilized Glucose Oxidase. ACS Omega 2018, 3, 667–676. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Prokhorov, E.; Bahena, D.; Esparza, R.; Meyyappan, M. Chitosan-Covered Pd@Pt Core–Shell Nanocubes for Direct Electron Transfer in Electrochemical Enzymatic Glucose Biosensor. ACS Omega 2017, 2, 1896–1904. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Mai, Z.; Zou, X.; Cai, P.; Mo, J. A novel glucose biosensor based on immobilization of glucose oxidase in chitosan on a glassy carbon electrode modified with gold-platinum alloy nanoparticles/multiwall carbon nanotubes. Anal. Biochem. 2007, 369, 71–79. [Google Scholar] [CrossRef]
- Tang, H.; Chen, J.; Yao, S.; Nie, L.; Deng, G.; Kuang, Y. Amperometric glucose biosensor based on adsorption of glucose oxidase at platinum nanoparticle-modified carbon nanotube electrode. Anal. Biochem. 2004, 331, 89–97. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, Y.; Liu, Z.; Rong, F.; Wang, Y.; Fu, D. Covalently immobilized biosensor based on gold nanoparticles modified TiO2 nanotube arrays. J. Electroanal. Chem. 2011, 650, 241–247. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research (CDER); Center for Veterinary Medicine (CVM) Bioanalytical Method Validation. Guidance for Industry; The Food and Drug Administration: White Oak Campus, MD, USA, 2018; pp. 1–41. [Google Scholar]
- AOAC International. AOAC Peer-Verified Methods Program, Manual on Policies and Procedures; AOAC International: Rockville, MD, USA, 1998. [Google Scholar]
- AOAC International. AOAC Definitions and Calculations of Horrat Values from Intralaboratory Data; AOAC International: Rockville, MD, USA, 2004. [Google Scholar]
- Tiwari, G.; Tiwari, R. Bioanalytical method validation: An updated review. Pharm. Methods 2010, 1, 25–38. [Google Scholar] [CrossRef]
- Vander Heyden, Y.; Nijhuis, A.; Smeyers-Verbeke, J.; Vandeginste, B.G.; Massart, D. Guidance for robustness/ruggedness tests in method validation. J. Pharm. Biomed. Anal. 2001, 24, 723–753. [Google Scholar] [CrossRef]
- Drozdz, R.; Naskalski, J.W.; Sznajd, J. Oxidation of amino acids and peptides in reaction with myeloperoxidase, chloride and hydrogen peroxide. Biochim. Biophys. Acta 1988, 957, 47–52. [Google Scholar] [CrossRef]
- Harris, J.M.; Reyes, C.; Lopez, G.P. Common Causes of Glucose Oxidase Instability in In Vivo Biosensing: A Brief Review. J. Diabetes Sci. Technol. 2013, 7, 1030–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumitraşcu, L.; Stănciuc, N.; Bahrim, G.E.; Ciumac, A.; Aprodu, I. pH and heat-dependent behaviour of glucose oxidase down to single molecule level by combined fluorescence spectroscopy and molecular modelling. J. Sci. Food Agric. 2016, 96, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Sepulveda, J. Challenges in Routine Clinical Chemistry Testing. In Accurate Results in the Clinical Laboratory; Elsevier: Amsterdam, The Netherlands, 2013; pp. 93–129. ISBN 9780124157835. [Google Scholar]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Baxamusa, S.H.; Montero, L.; Dubach, J.M.; Clark, H.A.; Borros, S.; Gleason, K.K. Protection of sensors for biological applications by photoinitiated chemical vapor deposition of hydrogel thin films. Biomacromolecules 2008, 9, 2857–2862. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, A.; Borrós, S. Comparison of two different plasma surface-modification techniques for the covalent immobilization of protein monolayers. Langmuir 2013, 29, 6645–6651. [Google Scholar] [CrossRef]
- Bilek, M.M.; McKenzie, D.R. Plasma modified surfaces for covalent immobilization of functional biomolecules in the absence of chemical linkers: Towards better biosensors and a new generation of medical implants. Biophys. Rev. 2010, 2, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Guilbault, G.G.; Kauffmann, J.-M.; Patriarche, G.J. Immobilized Enzyme Electrode as Biosensor. In Protein Immobilization. Fundamentals and Applications; Taylor, R.F., Ed.; Marcel Dekker: New York, NY, USA, 1991; pp. 209–262. [Google Scholar]





| Biosensors | Sensitivity/µA·mM−1 | LOD/mM | LOQ/mM | |
|---|---|---|---|---|
| Individual Values | Average | |||
| [38] | 9.76 | 8.53 ± 2.39 | 0.10 | 0.20 |
| Present work | 8.63 | 0.12 | 0.20 | |
| 5.15 | 0.14 | 0.21 | ||
| 10.58 | 0.10 | 0.19 | ||
| Biosensor | KMapp/mM | Reference |
|---|---|---|
| GCE-CNT/GOx | 5.95–14.50 | [45] |
| GCE/CS/CNT/Au-PtNPs/GOx | 5.20 | [47] |
| CNT/Pt/GOx/Nafion | 10.11 | [48] |
| Ti/TiO2NT/AuNPs/GOx | 7.2 | [49] |
| Ti/TiO2NTAs/ppHEMA-co-EGDA/pgPFM/GOx/Chitosan | 3.44 ± 0.67 | Present work |
| Method | [Glucose] ± s/M | RSD/% | Deviation/% |
|---|---|---|---|
| HPLC | 0.251 ± 0.001 | 0.3 | 4.8 |
| Biosensor | 0.263 ± 0.005 | 1.7 |
| Replicate | [Glucose]/M | [Glucose] Average ± s/M | RSD/% |
|---|---|---|---|
| 1 | 0.260 | 0.263 ± 0.005 | 1.7 |
| 2 | 0.268 | ||
| 3 | 0.261 |
| Day | [Glucose]/M | [Glucose] Average ± s/M | RSD/% |
|---|---|---|---|
| 1 | 0.266 | 0.262 ± 0.003 | 1.3 |
| 2 | 0.261 | ||
| 3 | 0.260 |
| Day | Biosensor | [Glucose]/M | [Glucose] Average ± s/M | RSD/% |
|---|---|---|---|---|
| 1 | 1 | 0.258 | 0.266 ± 0.006 | 2.4 |
| 2 | 2 | 0.270 | ||
| 3 | 3 | 0.268 |
| Sample | [Glucose]biosensor ± s/M | [Glucose]HPLC ± s/M | Deviation % |
|---|---|---|---|
| Orange Soft Drink | 0.263 ± 0.005 | 0.251 ± 0.001 | 4.8 |
| Lemon Soft Drink | 0.164 ± 0.001 | 0.150 ± 0.001 | 9.1 |
| Soya Sauce | 0.087 ± 0.002 | 0.096 ± 0.001 | −9.7 |
| Tomato Sauce | 0.559 ± 0.009 | 0.515 ± 0.005 | 8.4 |
| Yoghurt | 0.188 ± 0.002 | 0.174 ± 0.001 | 8.1 |
| Horchata | 0.038 ± 0.002 | 0.035 ± 0.001 | 8.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artigues, M.; Gilabert-Porres, J.; Texidó, R.; Borrós, S.; Abellà, J.; Colominas, S. Analytical Parameters of a Novel Glucose Biosensor Based on Grafted PFM as a Covalent Immobilization Technique. Sensors 2021, 21, 4185. https://doi.org/10.3390/s21124185
Artigues M, Gilabert-Porres J, Texidó R, Borrós S, Abellà J, Colominas S. Analytical Parameters of a Novel Glucose Biosensor Based on Grafted PFM as a Covalent Immobilization Technique. Sensors. 2021; 21(12):4185. https://doi.org/10.3390/s21124185
Chicago/Turabian StyleArtigues, Margalida, Joan Gilabert-Porres, Robert Texidó, Salvador Borrós, Jordi Abellà, and Sergi Colominas. 2021. "Analytical Parameters of a Novel Glucose Biosensor Based on Grafted PFM as a Covalent Immobilization Technique" Sensors 21, no. 12: 4185. https://doi.org/10.3390/s21124185
APA StyleArtigues, M., Gilabert-Porres, J., Texidó, R., Borrós, S., Abellà, J., & Colominas, S. (2021). Analytical Parameters of a Novel Glucose Biosensor Based on Grafted PFM as a Covalent Immobilization Technique. Sensors, 21(12), 4185. https://doi.org/10.3390/s21124185