Preliminary Investigation on Phytoplankton Dynamics and Primary Production Models in an Oligotrophic Lake from Remote Sensing Measurements
Abstract
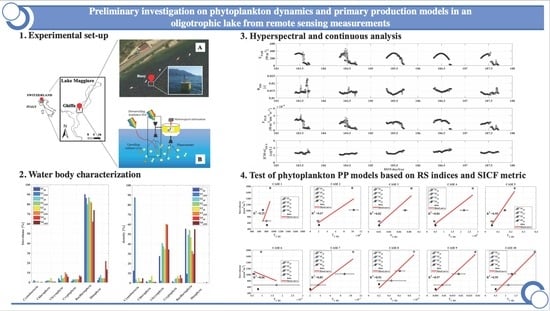
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Experiment Description
2.3. Laboratory Analysis
2.4. Fluorescence (FF) from Water Samples Analysis
2.5. Carbon Fixation Proxy (Φ’C) from Water Samples Analysis
2.6. In-Situ Continuous and Hyperspectral Measurements
2.6.1. Continuous Measurements Description
2.6.2. Fluorescence Metric (FFLH) from Hyperspectral Measurements
2.6.3. Spectral Indices from Hyperspectral Measurements
2.7. Phytoplankton Primary Production Models
3. Results
3.1. Characterization with the Water Samples Analysis
3.1.1. Phytoplankton Composition
3.1.2. Phytoplankton Dynamics
3.2. Spectral Measurements Analysis
3.3. Phytoplankton Primary Production Models Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruddick, K.G.; Voss, K.; Boss, E.; Castagna, A.; Frouin, R.; Gilerson, A.; Hieronymi, M.; Carol Johnson, B.; Kuusk, J.; Lee, Z.; et al. A review of protocols for fiducial reference measurements of water-leaving radiance for validation of satellite remote-sensing data over water. Remote Sens. 2019, 11, 2198. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, E.; Pratap, D.; Nagamani, P.V.; Rao, K.H.; Latha, T.P.; Choudhury, S.B. Time Series Analysis of Primary Productivity Along the East Coast of India using Oceansat-2 Ocean Colour Monitor (OCM). In Proceedings of the ISPRS Technical Commission VIII Symposium, Hyderabad, India, 9–14 December 2014; pp. 1049–1053. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, P.; Kiefer, D.A. Chlorophyll a fluorescence in phytoplankton: Relationship to photosynthesis and biomass. J. Plankton Res. 1985, 7, 715–731. [Google Scholar] [CrossRef]
- Smith, R.C.; Prezelin, B.B.; Bidigare, R.R.; KS, B. Bio-optical modeling of photosynthetic production in coastal waters. Limnol. Oceanogr. 1989, 34, 1524–1544. [Google Scholar] [CrossRef]
- Bukata, R.P.; Jerome, J.H.; Kondratyev, K.Y.; Pozdnyakov, D.V. Optical Properties and Remote Sensing of Inland and Coastal Waters; CPR Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Gitelson, A.A.; Gurlin, D.; Moses, W.J.; Barrow, T. A bio-optical algorithm for the remote estimation of the chlorophyll-a concentration in case 2 waters. Environ. Res. Lett. 2009, 4, 2–7. [Google Scholar] [CrossRef]
- Sun, Y.; Frankenberg, C.; Jung, M.; Joiner, J.; Guanter, L.; Köhler, P.; Magney, T. Overview of Solar-Induced chlorophyll Fluorescence (SIF) from the Orbiting Carbon Observatory-2: Retrieval, cross-mission comparison, and global monitoring for GPP. Remote Sens. Environ. 2018, 209, 808–823. [Google Scholar] [CrossRef]
- Mohammed, G.H.; Colombo, R.; Middleton, E.M.; Rascher, U.; van der Tol, C.; Nedbal, L.; Goulas, Y.; Pérez-Priego, O.; Damm, A.; Meroni, M.; et al. Remote sensing of solar-induced chlorophyll fluorescence (SIF) in vegetation: 50 years of progress. Remote Sens. Environ. 2019, 231, 111177. [Google Scholar] [CrossRef] [PubMed]
- Gower, J.F.R.; Doerffer, R.; Borstad, G.A. Interpretation of the 685nm peak in water-leaving radiance spectra in terms of fluorescence, absorption and scattering, and its observation by MERIS. Int. J. Remote Sens. 1999, 20, 1771–1786. [Google Scholar] [CrossRef]
- Gupana, R.S.; Odermatt, D.; Cesana, I.; Giardino, C.; Nedbal, L.; Damm, A. Remote Sensing of Environment Remote sensing of sun-induced chlorophyll-a fluorescence in inland and coastal waters: Current state and future prospects. Remote Sens. Environ. 2021, 262, 112482. [Google Scholar] [CrossRef]
- Butler, W.L. Energy Distribution in the Photochemical Apparatus of Photosynthesis. Annu. Rev. Plant Physio. 1978, 29, 345–378. [Google Scholar] [CrossRef]
- Duysens, L.N.M. Transfer and trapping of excitation energy in photosystem II. Chlorophyll Organ. Energy Transf. Photosynth. 1978, 7, 323–340. [Google Scholar]
- Zhao, F.; Li, R.; Verhoef, W.; Cogliati, S.; Liu, X.; Huang, Y.; Guo, Y.; Huang, J. Reconstruction of the full spectrum of solar-induced chlorophyll fluorescence: Intercomparison study for a novel method. Remote Sens. Environ. 2018, 219, 233–246. [Google Scholar] [CrossRef]
- Meroni, M.; Colombo, R. Leaf level detection of solar induced chlorophyll fluorescence by means of a subnanometer resolution spectroradiometer. Remote Sens. Environ. 2006, 103, 438–448. [Google Scholar] [CrossRef]
- Cogliati, S.; Celesti, M.; Cesana, I.; Miglietta, F.; Genesio, L.; Julitta, T.; Schuettemeyer, D.; Drusch, M.; Rascher, U.; Jurado, P.; et al. A spectral fitting algorithm to retrieve the fluorescence spectrum from canopy radiance. Remote Sens. 2019, 11, 1840. [Google Scholar] [CrossRef] [Green Version]
- Gilerson, A.; Zhou, J.; Hlaing, S.; Ioannou, I.; Schalles, J.; Gross, B.; Ahmed, S. Fluorescence component in the reflectance spectra from coastal waters. Dependence on water composition. Opt. Express 2007, 15, 15702–15721. [Google Scholar] [CrossRef]
- Mobley, C.; Stramsky, D.; Bissett, W.; Boss, E. Optical Modeling of Ocean Water. Oceanography 2004, 17, 60–67. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Westberry, T.K.; Boss, E.S.; O’Malley, R.T.; Siegel, D.A.; Wiggert, J.D.; Mahowald, N. Satellite-detected fluorescence reveals global physiology of ocean phytoplankton. Biogeosciences 2009, 6, 779–794. [Google Scholar] [CrossRef] [Green Version]
- Bresciani, M.; Giardino, C.; Hommersom, A.; Manca, D.; Julitta, T.; Cesana, I.; Valentina, D.B.; Rosalba, P. Analysis Of High Frequency Hyperspectral Remote Sensing Reflectances From Autonomous In Situ Sensors Deployed In Lakes. In Proceedings of the 11th EARSeL SIG IS Workshop, Brno, Czech Republic, 6–8 February 2019. [Google Scholar]
- Abbott, M.R.; Letelier, R.M. Chlorophyll fluorescence (MODIS product number 20) (ATBD 22). Ocean. Color Web Page 1999, 20, 1–42. [Google Scholar]
- Deng, Y.; Zhang, Y.; Li, D.; Shi, K.; Zhang, Y. Temporal and Spatial Dynamics of Phytoplankton Primary Production in Lake Taihu Derived from MODIS Data. Remote Sens. 2017, 9, 195. [Google Scholar] [CrossRef]
- Monteith, J.L. Solar Radiation and Productivity in Tropical Ecosystems. J. Appl. Ecol. 1972, 9, 747–766. [Google Scholar] [CrossRef] [Green Version]
- Rossini, M.; Meroni, M.; Migliavacca, M.; Manca, G.; Cogliati, S.; Busetto, L.; Picchi, V.; Cescatti, A.; Seufert, G.; Colombo, R. High resolution field spectroscopy measurements for estimating gross ecosystem production in a rice field. Agric. Forest Meteo. 2010, 150, 1283–1296. [Google Scholar] [CrossRef]
- Meroni, M.; Picchi, V.; Rossini, M.; Cogliati, S.; Panigada, C.; Nali, C.; Lorenzini, G.; Colombo, R. Leaf level early assessment of ozone injuries by passive fluorescence and photochemical reflectance index. Int. J. Remote Sens. 2008, 29, 5409–5422. [Google Scholar] [CrossRef]
- Damm, A.; Elber, J.; Erler, A.; Gioli, B.; Hamdi, K.; Hutjes, R.; Kosvancova, M.; Meroni, M.; Miglietta, F.; Moersch, A.; et al. Remote sensing of sun-induced fluorescence to improve modeling of diurnal courses of gross primary production (GPP). Global Chang. Biol. 2010, 16, 171–186. [Google Scholar] [CrossRef]
- Meroni, M.; Rossini, M.; Picchi, V.; Panigada, C.; Cogliati, S.; Nali, C.; Colombo, R. Assessing steady-state fluorescence and PRI from hyperspectral proximal sensing as early indicators of plant stress: The case of ozone exposure. Sensors 2008, 8, 1740–1754. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, D.A.; Chamberlin, W.S.; Booth, C.R. Natural fluorescence of chlorophyll a: Relationship to photosynthesis and chlorophyll concentration in the western South Pacific gyre. Limnol. Oceanogr. 1989, 34, 868–881. [Google Scholar] [CrossRef]
- Morrison, J.R. In situ determination of the quantum yield of phytoplankton chlorophyll a fluorescence: A simple algorithm, observations, and a model. Limnol. Oceanogr. 2003, 48, 618–631. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Chen, C.; Sun, Z.; Tang, S.; Song, X.; Yang, C.; Tian, L.; Liu, F. Estimation of the Primary Productivity in Pearl River Estuary Using MODIS Data. Estuaries Coasts 2015, 38, 506–518. [Google Scholar] [CrossRef]
- Kemili, P.; Putri, M.R. Estimation of primary productivity in Banda Sea using the vertical distribution model. AIP Conf. Proc. 2014, 1589, 389–393. [Google Scholar] [CrossRef]
- Bergamino, N.; Horion, S.; Stenuite, S.; Cornet, Y.; Loiselle, S.; Plisnier, P.D.; Descy, J.P. Spatio-temporal dynamics of phytoplankton and primary production in Lake Tanganyika using a MODIS based bio-optical time series. Remote Sens. Environ. 2010, 114, 772–780. [Google Scholar] [CrossRef]
- Barbanti, L.; Ambrosetti, W. The physical limnology of Lago Maggiore: A review. Mem. Inst. Ital. Idrobiol. 1989, 46, 41–68. [Google Scholar]
- Commissione Internazionale per la Protezione delle Acque Italo-Svizzere. Indagini su DDT e Sostanze Pericolose Nell’Ecosistema Del Lago Maggiore; Rapporto Annuale 2015 e Finale 2013–2015; Pallanza: Verbania, Italy, 2015. (in Italian) [Google Scholar]
- Salmaso, N.; Mosello, R. Limnological research in the deep southern subalpine lakes: Synthesis, directions and perspectives. Adv. Oceanogr. Limnol. 2010, 1, 29–66. [Google Scholar] [CrossRef]
- Ruggiu, D.; Morabito, G.; Panzani, P.; Pugnetti, A. Trends and relations among basic phytoplankton characteristics in the course of the long-term oligotrophication of Lake Maggiore (Italy). Hydrobiologia 1998, 370, 243–257. [Google Scholar] [CrossRef]
- Marchetto, A.; Lami, A.; Musazzi, S.; Massaferro, J.; Langone, L.; Guilizzoni, P. Lake Maggiore (N. Italy) trophic history: Fossil diatom, plant pigments, and chironomids, and comparison with long-term limnological data. Quat. Int. 2004, 113, 97–110. [Google Scholar] [CrossRef]
- Morabito, G.; Rogora, M.; Austoni, M.; Ciampittiello, M. Could the extreme meteorological events in Lake Maggiore watershed determine a climate-driven eutrophication process? Hydrobiologia 2018, 824, 163–175. [Google Scholar] [CrossRef]
- De Bernardi, R.; Giussani, G.; Manca, M.; Ruggiu, D. Long-term dynamics of plankton communities in Lago Maggiore (N. Italy). Verh. Int. Ver. Limnol. 1988, 23, 729–733. [Google Scholar] [CrossRef]
- Manca, M.; Calderoni, A.; Mosello, R. Limnological research in Lago Maggiore: Studies on hydrochemistry and plankton. Mem. Inst. Ital. Idrobiol. 1992, 50, 171–200. [Google Scholar]
- Luhtala, H.; Tolvanen, H. Optimizing the use of Secchi depth as a proxy for euphotic depth in coastal waters: An empirical study from the Baltic Sea. ISPRS Int. J. Geo Inf. 2013, 2, 1153–1168. [Google Scholar] [CrossRef]
- Bresciani, M.; Giardino, C.; Rosaria, L.; Matta, E.; Cazzaniga, I.; Pinardi, M.; Lami, A.; Austoni, M.; Viaggiu, E.; Congestri, R.; et al. Earth observation for monitoring and mapping of cyanobacteria blooms. Case studies on five Italian lakes. J. Limnol. 2016, 76, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Lorenzen, C.J. Determination of Chlorophyll and Pheo-Pigments: Spectrophotometric Equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Guilizzoni, P.; Marchetto, A.; Lami, A.; Gerli, S.; Musazzi, S. Use of sedimentary pigments to infer past phosphorus concentration in lakes. J. Paleolimnol. 2011, 45, 433–445. [Google Scholar] [CrossRef]
- Strömbeck, N.; Pierson, D.C. The effects of variability in the inherent optical properties on estimations of chlorophyll a by remote sensing in Swedish freshwaters. Sci. Total Environ. 2001, 268, 123–137. [Google Scholar] [CrossRef]
- Kishino, M.; Takahashi, M.; Okami, N.; Ichimura, S. Estimation of the spectral absorption coefficients of phytoplankton in the sea. Bull. Mar. Sci. 1985, 37, 634–642. [Google Scholar]
- Trüper, H.G.; Yentsch, C.S. Use of glass fiber filters for the rapid preparation of in vivo absorption spectra of photosynthetic bacteria. J. Bacteriol. 1967, 94, 1255–1256. [Google Scholar] [CrossRef] [Green Version]
- Kirk, J.T. Light and Photosynthesis in Aquatic Ecosystems, 3rd ed.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik. Mitt. Int. Verein. Limnol. 1958, 9, 1–38. [Google Scholar]
- Remelli, W.; Santabarbara, S. Excitation and emission wavelength dependence of fluorescence spectra in whole cells of the cyanobacterium Synechocystis sp. PPC6803: Influence on the estimation of Photosystem II maximal quantum efficiency. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Santabarbara, S.; Tibiletti, T.; Remelli, W.; Caffarri, S. Kinetics and heterogeneity of energy transfer from light harvesting complex II to photosystem i in the supercomplex isolated from Arabidopsis. Phys. Chem. Chem. Phys. 2017, 19, 9210–9222. [Google Scholar] [CrossRef]
- Mishra, D.R.; Narumalani, S.; Rundquist, D.; Lawson, M. Characterizing the vertical diffuse attenuation coefficient for downwelling irradiance in coastal waters: Implications for water penetration by high resolution satellite data. ISPRS J. Photogram. Remote Sens. 2005, 60, 48–64. [Google Scholar] [CrossRef]
- Zibordi, G.; Ruddick, K.; Ansko, I.; Moore, G.; Kratzer, S.; Icely, J.; Reinart, A. In situ determination of the remote sensing reflectance: An inter-comparison. Ocean. Sci. Discuss. 2012, 9, 787–833. [Google Scholar] [CrossRef] [Green Version]
- Austin, R.W. The remote sensing of spectral radiance from below the ocean surface. Opt. Asp. Oceanogr. 1974, 317–344. [Google Scholar]
- Cogliati, S.; Rossini, M.; Julitta, T.; Meroni, M.; Schickling, A.; Burkart, A.; Pinto, F.; Rascher, U.; Colombo, R. Continuous and long-term measurements of reflectance and sun-induced chlorophyll fluorescence by using novel automated field spectroscopy systems. Remote Sens. Environ. 2015, 164, 270–281. [Google Scholar] [CrossRef]
- Gower, J.F. Observations of in-situ fluorescence of chlorophyll-a in Saanich inlet. Bound. Layer Meteo. 1980, 18, 235–245. [Google Scholar] [CrossRef]
- Letelier, R.M.; Abbott, M.R. An analysis of chlorophyll fluorescence algorithms for the moderate resolution imaging spectrometer (MODIS). Remote Sens. Environ. 1996, 58, 215–223. [Google Scholar] [CrossRef]
- Xing, X.G.; Zhao, D.Z.; Liu, Y.G.; Yang, J.H.; Xiu, P.; Wang, L. An overview of remote sensing of chlorophyll fluorescence. Ocean Sci. J. 2007, 42, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Dall’Olmo, G.; Gitelson, A.A.; Rundquist, D.C. Towards a unified approach for remote estimation of chlorophyll-a in both terrestrial vegetation and turbid productive waters. Geophy. Res. Lett. 2003, 30, 8–11. [Google Scholar] [CrossRef] [Green Version]
- Gilerson, A.A.; Gitelson, A.A.; Zhou, J.; Gurlin, D.; Moses, W.; Ioannou, I.; Ahmed, S.A. Algorithms for remote estimation of chlorophyll-a in coastal and inland waters using red and near infrared bands. Opt. Express 2010, 18, 24109. [Google Scholar] [CrossRef] [Green Version]
- Gower, J.F.R.; Brown, L.; Borstad, G.A. Observation of chlorophyll fluorescence in west coast waters of Canada using the MODIS satellite sensor. Can. J. Remote Sens. 2004, 30, 17–25. [Google Scholar] [CrossRef]
- Kritten, L.; Preusker, R.; Fischer, J. A new retrieval of sun-induced chlorophyll fluorescence in water from ocean colour measurements applied on olci l-1b and l-2. Remote Sens. 2020, 12, 3949. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Maritorena, S.; Siegel, D.A.; O’Brien, M.C.; Toole, D.; Mitchell, G.B.; Kahru, M.; Chavez, F.P.; Strutton, P.; Cota, G.F.; et al. Ocean color chlorophyll a algorithms for SeaWiFS, OC2, and OC4: Version 4. SeaWiFS Postlaunch Calibration Valid. Anal. 2000, 3, 8–22. [Google Scholar]
- Morabito, G.; Oggioni, A.; Caravati, E.; Panzani, P. Seasonal morphological plasticity of phytoplankton in Lago Maggiore (N. Italy). Hydrobiologia 2007, 578, 47–57. [Google Scholar] [CrossRef]
- Morabito, G.; Ruggiu, D.; Panzani, P. Recent dynamics (1995–1999) of the phytoplankton assemblages in Lago Maggiore as a basic tool for defining association patterns in the Italian deep lakes. J. Limnol. 2002, 61, 129–145. [Google Scholar] [CrossRef] [Green Version]
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Smetacek, V. Diatoms and the ocean carbon cycle. Protist 1999, 150, 25–32. [Google Scholar] [CrossRef]
- Znachor, P.; Šimek, K.; Nedoma, J. Bacterial colonization of the freshwater planktonic diatom Fragilaria crotonensis. Aquat. Microb. Ecol. 2012, 66, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Znachor, P.; Nedoma, J. Application of the PDMPO technique in studying silica deposition in natural populations of Fragilaria crotonensis (Bacillariophyceae) at different depths in a eutrophic reservoir. J. Phycol. 2008, 44, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.S. Vegetation Processes in the Pelagic. A Model for Ecosystem Theory; ECI: Oldendorf, Germany, 1997. [Google Scholar]
- Morabito, G.; Oggioni, A.; Panzani, P. Phytoplankton assemblage at equilibrium in large and deep subalpine lakes: A case study from Lago Maggiore (N. Italy). Hydrobiologia 2003, 502, 37–48. [Google Scholar] [CrossRef]
- Reynolds, C.S. The development of perceptions of aquatic eutrophication and its control. Ecohydrol. Hydrobiol. 2003, 3, 149–163. [Google Scholar]
- Horton, P.; Ruban, A.V.; Walters, R.G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 655–684. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Caffarri, S.; Tibiletti, T.; Jennings, R.; Santabarbara, S. A Comparison Between Plant Photosystem I and Photosystem II Architecture and Functioning. Curr. Protein Pept. Sci. 2014, 15, 296–331. [Google Scholar] [CrossRef]
- Reynolds, C.S. Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Bresciani, M.; Brewin, R.; Brivio, P.A.; Giardino, C.; Gomarasca, M.A.; Hunter, P.; De Keukelare, L.; Kiselev, V.; Klinger, P.; Knaps, E.; et al. INFORM_D5.14_v1.0 Inform Algorithms Theoretical Basis Document. 2016, pp. 1–158. Available online: https://inform.vgt.vito.be/files/documents/INFORM_D5.14_v1.0.pdf (accessed on 6 June 2021).
- Bootsma, H.A.; Hecky, R.E. Conservation of the African Great Lakes: A limnological perspective. Conser. Bio. 1993, 7, 644–656. [Google Scholar] [CrossRef]
- Drusch, M.; Moreno, J.; Del Bello, U.; Franco, R.; Goulas, Y.; Huth, A.; Middleton, E.M.; Mohammed, G.; Rascher, U.; Verhoef, W.; et al. The FLuorescence Explorer Mission Concept—ESA’s Earth Explorer 8. IEEE Trans. Geosci. Remote Sens. 2017, 55, 1273–1284. [Google Scholar] [CrossRef]
- Barnes, M.K.; Tilstone, G.H.; Smyth, T.J.; Suggett, D.J.; Astoreca, R.; Lancelot, C.; Kromkamp, J.C. Absorption-based algorithm of primary production for total and size-fractionated phytoplankton in coastal waters. Marine Ecol. Prog. Series 2014, 504, 73–89. [Google Scholar] [CrossRef]









| CASE ID | ΦC | FA | CASE ID | ΦC | FA |
|---|---|---|---|---|---|
| 1 | constant | [Chl-a]OC4 | 6 | FFLH/FA | [Chl-a]OC4 |
| 2 | constant | EPAR | 7 | FFLH/FA | EPAR |
| 3 | constant | FFLH | 8 | FFLH/FA | FFLH |
| 4 | constant | [Chl-a]OC4·EPAR | 9 | FFLH/FA | [Chl-a]OC4·EPAR |
| 5 | constant | Chl-a]OC4·FFLH | 10 | FFLH/FA | Chl-a]OC4·FFLH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesana, I.; Bresciani, M.; Cogliati, S.; Giardino, C.; Gupana, R.; Manca, D.; Santabarbara, S.; Pinardi, M.; Austoni, M.; Lami, A.; et al. Preliminary Investigation on Phytoplankton Dynamics and Primary Production Models in an Oligotrophic Lake from Remote Sensing Measurements. Sensors 2021, 21, 5072. https://doi.org/10.3390/s21155072
Cesana I, Bresciani M, Cogliati S, Giardino C, Gupana R, Manca D, Santabarbara S, Pinardi M, Austoni M, Lami A, et al. Preliminary Investigation on Phytoplankton Dynamics and Primary Production Models in an Oligotrophic Lake from Remote Sensing Measurements. Sensors. 2021; 21(15):5072. https://doi.org/10.3390/s21155072
Chicago/Turabian StyleCesana, Ilaria, Mariano Bresciani, Sergio Cogliati, Claudia Giardino, Remika Gupana, Dario Manca, Stefano Santabarbara, Monica Pinardi, Martina Austoni, Andrea Lami, and et al. 2021. "Preliminary Investigation on Phytoplankton Dynamics and Primary Production Models in an Oligotrophic Lake from Remote Sensing Measurements" Sensors 21, no. 15: 5072. https://doi.org/10.3390/s21155072
APA StyleCesana, I., Bresciani, M., Cogliati, S., Giardino, C., Gupana, R., Manca, D., Santabarbara, S., Pinardi, M., Austoni, M., Lami, A., & Colombo, R. (2021). Preliminary Investigation on Phytoplankton Dynamics and Primary Production Models in an Oligotrophic Lake from Remote Sensing Measurements. Sensors, 21(15), 5072. https://doi.org/10.3390/s21155072