Use of Amperometric and Potentiometric Probes in Scanning Electrochemical Microscopy for the Spatially-Resolved Monitoring of Severe Localized Corrosion Sites on Aluminum Alloy 2098-T351
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Solutions
2.2. Instrumentation
3. Results and Discussion
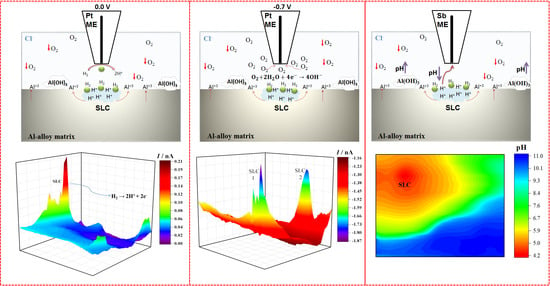
3.1. SECM Characterization of a Model Mg-Al Galvanic Pair
3.2. SECM Characterization of the 2098-T351 Al-Alloy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warner, T. Recently-developed aluminium solutions for aerospace applications. Mater. Sci. Forum 2006, 519–521, 1271–1278. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Huang, W.; Thompson, G.E.; Zhang, X.; Luo, C.; Sun, Z. Localized corrosion in AA2099-T83 aluminum-lithium alloy: The role of intermetallic particles. Mater. Chem. Phys. 2015, 161, 201–210. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Liao, Y.; Yi, Y.; Wu, H.; Wang, Z.; Huang, W. Localised corrosion in AA 2099-T83 aluminium-lithium alloy: The role of grain orientation. Corros. Sci. 2016, 107, 41–48. [Google Scholar] [CrossRef]
- Salem, H.G.; Lyons, J.S. Effect of equal channel angular extrusion on the microstructure and superplasticity of an Al-Li alloy. J. Mater. Eng. 2002, 11, 384–391. [Google Scholar] [CrossRef]
- Lequeu, P.; Smith, K.P.; Daniélou, A. Aluminum-copper-lithium alloy 2050 developed for medium to thick plate. J. Mater. Eng. 2010, 19, 841–847. [Google Scholar] [CrossRef] [Green Version]
- De, P.S.; Mishra, R.S.S.; Baumann, J.A.A. Characterization of high cycle fatigue behavior of a new generation aluminum lithium alloy. Acta Mater. 2011, 59, 5946–5960. [Google Scholar] [CrossRef]
- Milagre, M.X.; Donatus, U.; Machado, C.S.C.; Araujo, J.V.S.; da Silva, R.M.P.; de Viveiros, B.V.G.; Astarita, A.; Costa, I. Comparison of the corrosion resistance of an Al–Cu alloy and an Al–Cu–Li alloy. Corros. Eng. Sci. Technol. 2019, 54, 402–412. [Google Scholar] [CrossRef]
- Milagre, M.X.; Mogili, N.V.V.; Donatus, U.; Giorjão, R.A.R.; Terada, M.; Araujo, J.V.S.; Machado, C.S.C.; Costa, I. On the microstructure characterization of the AA2098-T351 alloy welded by FSW. Mater. Charact. 2018, 140, 233–246. [Google Scholar] [CrossRef]
- Donatus, U.; Ferreira, R.O.; Mogili, N.V.V.; de Viveiros, B.V.G.; Milagre, M.X.; Costa, I. Corrosion and anodizing behaviour of friction stir weldment of AA2198-T851 Al-Cu-Li alloy. Mater. Chem. Phys. 2018, 219, 493–511. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Moran, J.P.; Stoner, G.E. Localized corrosion behavior of alloy 2090- The role of microstructural heterogeneity. Corrosion 1990, 46, 610–617. [Google Scholar] [CrossRef]
- Proton, V.; Alexis, J.; Andrieu, E.; Blanc, C.; Delfosse, J.; Lacroix, L.; Odemer, G. Influence of post-welding heat treatment on the corrosion behavior of a 2050-T3 aluminum-copper-lithium alloy friction stir welding joint. J. Electrochem. Soc. 2011, 158, C139–C147. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhou, X.; Huang, W.; Liao, Y.; Chen, X.; Zhang, X.; Thompson, G.E. Crystallographic defects induced localised corrosion in AA2099-T8 aluminium alloy. Corros. Eng. Sci. Technol. 2015, 50, 420–424. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Hashimoto, T.; Lindsay, J.; Ciuca, O.; Luo, C.; Sun, Z.; Zhang, X.; Tang, Z. The influence of grain structure on the corrosion behaviour of 2A97-T3 Al-Cu-Li alloy. Corros. Sci. 2017, 116, 14–21. [Google Scholar] [CrossRef]
- Bard, A.J.; Denuault, G.; Lee, C.; Mandler, D.; Wipf, D.O. Scanning electrochemical microscopy—a new technique for the characterization and modification of surfaces. Acc. Chem. Res. 1990, 23, 357–363. [Google Scholar] [CrossRef]
- Fan, F.-R.F.; Demaille, C. Preparation of tips for scanning electrochemical microscopy. In Scanning Electrochemical Microscopy, 2nd ed.; Bard, A.J., Mirkin, M.V., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 25–51. [Google Scholar]
- Jensen, M.B.; Tallman, D.E. Application of SECM to corrosion studies. In Electroanalytical Chemistry—A Series of Advances; Bard, A.J., Zoski, C.G., Eds.; CRC Press: Boca Raton, FL, USA, 2012; Volume 24, pp. 171–286. [Google Scholar]
- Payne, N.A.; Stephens, L.I.; Mauzeroll, J. The application of scanning electrochemical microscopy to corrosion research. Corrosion 2017, 73, 759–780. [Google Scholar] [CrossRef]
- Xia, D.-H.; Wang, J.; Wu, Z.; Qin, Z.; Xu, L.; Hu, W.; Behnamian, Y.; Luo, J.-L. Sensing corrosion within an artificial defect in organic coating using SECM. Sensor. Actuat. B-Chem. 2019, 280, 235–242. [Google Scholar] [CrossRef]
- Lamaka, S.; Souto, R.M.; Ferreira, M.G.S. In-situ visualization of local corrosion by scanning ion-selective electrode technique (SIET). In Microscopy: Science, Technology, Applications and Education; Méndez-Vilas, A., Díaz, J., Eds.; Formatex: Badajoz, Spain, 2010; Volume 3, pp. 2162–2173. [Google Scholar]
- Nazarov, V.A.; Taryba, M.G.; Zdrachek, E.A.; Andronchyk, K.A.; Egorov, V.V.; Lamaka, S.V. Sodium- and chloride-selective microelectrodes optimized for corrosion studies. J. Electroanal. Chem. 2013, 706, 13–24. [Google Scholar] [CrossRef]
- Izquierdo, J.; Nagy, L.; Bitter, I.; Souto, R.M.; Nagy, G. Potentiometric scanning electrochemical microscopy for the local characterization of the electrochemical behaviour of magnesium-based materials. Electrochim. Acta 2013, 87, 283–293. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Karavai, O.V.; Bastos, A.C.; Zheludkevich, M.L.; Ferreira, M.G.S. Monitoring local spatial distribution of Mg2+, pH and ionic currents. Electrochem. Commun. 2008, 10, 259–262. [Google Scholar] [CrossRef]
- Izquierdo, J.; Nagy, L.; Varga, Á.; Santana, J.J.; Nagy, G.; Souto, R.M. Spatially resolved measurement of electrochemical activity and pH distributions in corrosion processes by scanning electrochemical microscopy using antimony microelectrode tips. Electrochim. Acta 2011, 56, 8846–8850. [Google Scholar] [CrossRef]
- Alvarez-Pampliega, A.; Taryba, M.G.; Van den Bergh, K.; De Strycker, J.; Lamaka, S.V.; Terryn, H. Study of local Na+ and Cl− distributions during the cut-edge corrosion of aluminum rich metal-coated steel by scanning vibrating electrode and micro-potentiometric techniques. Electrochim. Acta 2013, 102, 319–327. [Google Scholar] [CrossRef]
- Souto, R.M.; Kiss, A.; Izquierdo, J.; Nagy, L.; Bitter, I.; Nagy, G. Spatially-resolved imaging of concentration distributions on corroding magnesium-based materials exposed to aqueous environments by SECM. Electrochem. Commun. 2013, 26, 25–28. [Google Scholar] [CrossRef]
- Fernández-Pérez, B.M.; Izquierdo, J.; González, S.; Souto, R.M. Scanning electrochemical microscopy studies for the characterization of localized corrosion reactions at cut edges of coil-coated steel. J. Solid State Electrochem. 2014, 18, 2983–2992. [Google Scholar] [CrossRef]
- Marques, A.G.; Taryba, M.G.; Panão, A.S.; Lamaka, S.V.; Simões, A.M. Application of scanning electrode techniques for the evaluation of iron–zinc corrosion in nearly neutral chloride solutions. Corros. Sci. 2016, 104, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Pérez, B.M.; Izquierdo, J.; Santana, J.J.; González, S.; Souto, R.M. Scanning electrochemical microscopy studies for the characterization of localized corrosion reactions at cut edges of painted galvanized steel as a function of solution pH. Int. J. Electrochem. Sci. 2015, 10, 10145–10156. [Google Scholar]
- Ogle, K.; Morel, S.; Jacquet, D. Observation of self-healing functions on the cut edge of galvanized steel using SVET and pH microscopy. J. Electrochem. Soc. 2006, 153, B1–B5. [Google Scholar] [CrossRef]
- Zdrachek, E.A.; Karotkaya, A.G.; Nazarov, V.A.; Andronchyk, K.A.; Stanishevskii, L.S.; Egorov, V.V.; Taryba, M.G.; Snihirova, D.; Kopylovich, M.; Lamaka, S.V. H+-selective microelectrodes with optimized measuring range for corrosion studies. Sensor. Actuat. B-Chem. 2015, 207, 967–975. [Google Scholar] [CrossRef]
- Davoodi, A.; Pan, J.; Leygraf, C.; Norgren, S. Probing of local dissolution of Al-alloys in chloride solutions by AFM and SECM. Appl. Surf. Sci. 2006, 252, 5499–5503. [Google Scholar] [CrossRef]
- Jensen, M.B.; Guerard, A.; Tallman, D.E.; Bierwagen, G.P. Studies of electron transfer at aluminum alloy surfaces by scanning electrochemical microscopy. J. Electrochem. Soc. 2008, 155, C324–C332. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Dong, C.; Xiao, K.; Li, T. Corrosion behavior of aluminum alloys in Na2SO4 solution using the scanning electrochemical microscopy technique. Int. J. Miner. Metall. Mater. 2009, 16, 84–88. [Google Scholar] [CrossRef]
- Liu, W.; Singh, A.; Lin, Y.; Ebenso, E.E.; Tianhan, G.; Ren, C. Corrosion inhibition of Al-alloy in 3.5% NaCl solution by a natural inhibitor: An electrochemical and surface study. Int. J. Electrochem. Sci. 2014, 9, 5560–5573. [Google Scholar]
- Sidane, D.; Bousquet, E.; Devos, O.; Puiggali, M.; Touzet, M.; Vivier, V. Local electrochemical study of friction stir welded aluminum alloy assembly. J. Electroanal. Chem. 2014, 737, 206–211. [Google Scholar] [CrossRef]
- Lafouresse, M.C.; de Bonfils-Lahovary, M.L.; Laffont, L.; Blanc, C. Hydrogen mapping in an aluminum alloy using an alternating current scanning electrochemical microscope (AC-SECM). Electrochem. Commun. 2017, 80, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Bastos, A.C.; Simões, A.M.; González, S.; González-García, Y.; Souto, R.M. Imaging concentration profiles of redox-active species in open-circuit corrosion processes with the scanning electrochemical microscope. Electrochem. Commun. 2004, 6, 1212–1215. [Google Scholar] [CrossRef]
- Jamali, S.S.; Moulton, S.E.; Tallman, D.E.; Forsyth, M.; Weber, J.; Wallace, G.G. Applications of scanning electrochemical microscopy (SECM) for local characterization of AZ31 surface during corrosion in a buffered media. Corros. Sci. 2014, 86, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Dauphin-Ducharme, P.; Asmussen, R.M.; Tefashe, U.M.; Danaie, M.; Binns, W.J.; Jakupi, P.; Botton, G.A.; Shoesmith, D.W.; Mauzeroll, J. Local hydrogen fluxes correlated to microstructural features of a corroding sand cast AM50 magnesium alloy. J. Electrochem. Soc. 2014, 161, C557–C564. [Google Scholar] [CrossRef]
- Izquierdo, J.; Fernández-Pérez, B.M.; Filotás, D.; Őri, Z.; Kiss, A.; Martín-Gómez, R.T.; Nagy, L.; Nagy, G.; Souto, R.M. Imaging of concentration distributions and hydrogen evolution on corroding magnesium exposed to aqueous environments using scanning electrochemical microscopy. Electroanalysis 2016, 28, 2354–2366. [Google Scholar] [CrossRef]
- Mena-Morcillo, E.; Veleva, L.; Wipf, D. In situ investigation of the initial stages of AZ91D magnesium alloy biodegradation in simulated body fluid. Int. J. Electrochem. Sci. 2018, 13, 5141–5150. [Google Scholar] [CrossRef]
- Souto, R.M.; Fernández-Mérida, L.; González, S. SECM imaging of interfacial processes in defective organic coatings applied on metallic substrates using oxygen as redox mediator. Electroanalysis 2009, 21, 2640–2646. [Google Scholar] [CrossRef]
- Thomas, S.; Izquierdo, J.; Birbilis, N.; Souto, R.M. Possibilities and limitations of scanning electrochemical microscopy of Mg and Mg alloys. Corrosion 2015, 71, 171–183. [Google Scholar] [CrossRef]
- Snihirova, D.; Taryba, M.; Lamaka, S.V.; Montemor, M.F. Corrosion inhibition synergies on a model Al-Cu-Mg sample studied by localized scanning electrochemical techniques. Corros. Sci. 2016, 112, 408–417. [Google Scholar] [CrossRef]
- Bastos, A.C.; Simões, A.M.; González, S.; González-García, Y.; Souto, R.M. Application of the scanning electrochemical microscope to the examination of organic coatings on metallic substrates. Prog. Org. Coat. 2005, 53, 177–182. [Google Scholar] [CrossRef]
- Araujo, J.V.S.; Donatus, U.; Queiroz, F.M.; Terada, M.; Milagre, M.X.; de Alencar, M.C.; Costa, I. On the severe localized corrosion susceptibility of the AA2198-T851 alloy. Corros. Sci. 2018, 133, 132–140. [Google Scholar] [CrossRef]
- Donatus, U.; Araujo, J.V.S.; Machado, C.S.C.; Mogili, N.V.V.; Antunes, R.A.; Costa, I. The effect of manufacturing process induced near-surface deformed layer on the corrosion behaviour of AA2198-T851 Al–Cu–Li alloy. Corros. Eng. Sci. Technol. 2018, 54, 205–215. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Moran, J.P.; Stoner, G.E. Electrochemical behavior of the T1 (Al2CuLi) intermetallic compound and its role in localized corrosion of Al-2% Li-3% Cu alloys. Corrosion 1994, 50, 120–130. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Meng, X.; Huang, W.; Liao, Y. Influence of thermomechanical treatments on localized corrosion susceptibility and propagation mechanism of AA2099 Al−Li alloy. T. Nonferr. Metal Soc. 2016, 26, 1472–1481. [Google Scholar] [CrossRef]
- Luo, C.; Albu, S.P.; Zhou, X.; Sun, Z.; Zhang, X.; Tang, Z.; Thompson, G.E. Continuous and discontinuous localized corrosion of a 2xxx aluminium-copper- lithium alloy in sodium chloride solution. J. Alloys Compd. 2016, 658, 61–70. [Google Scholar] [CrossRef]
- Guérin, M.; Alexis, J.; Andrieu, E.; Laffont, L.; Lefebvre, W.; Odemer, G.; Blanc, C. Identification of the metallurgical parameters explaining the corrosion susceptibility in a 2050 aluminium alloy. Corros. Sci. 2016, 102, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhou, X.; Hashimoto, T.; Liu, B.; Luo, C.; Sun, Z.; Tang, Z.; Lu, F.; Ma, Y. Corrosion behaviour of 2A97-T6 Al-Cu-Li alloy: The influence of non-uniform precipitation. Corros. Sci. 2018, 132, 1–8. [Google Scholar] [CrossRef]
- Araujo, J.V.S.; Bugarin, A.F.S.; Donatus, U.; Machado, C.S.C.; Queiroz, F.M.; Terada, M.; Astarita, A.; Costa, I. Thermomechanical treatment and corrosion resistance correlation in the AA2198 Al-Cu-Li alloy. Corros. Eng. Sci. Technol. 2019, 54, 575–586. [Google Scholar] [CrossRef]
- Donatus, U.; Terada, M.; Ramirez, C.; Martins, F.; Bugarin, A.F.S.; Costa, I. On the AA2198-T851 alloy microstructure and its correlation with localized corrosion behaviour. Corros. Sci. 2018, 131, 300–309. [Google Scholar] [CrossRef]
- da Silva, R.M.P.; Milagre, M.X.; de Oliveira, L.A.; Donatus, U.; Antunes, R.A.; Costa, I. The local electrochemical behavior of the AA2098-T351 and surface preparation effects investigated by scanning electrochemical microscopy. Surf. Interface Anal. 2019, 51, 982–992. [Google Scholar] [CrossRef]
- Tsuyuki, C.; Yamanaka, A.; Ogimoto, Y. Phase-field modeling for pH-dependent general and pitting corrosion of iron. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Silva, E.L.; Lamaka, S.V.; Mei, D.; Zheludkevich, M.L. The reduction of dissolved oxygen during magnesium corrosion. ChemistryOpen 2018, 7, 664–668. [Google Scholar] [CrossRef]
- Donatus, U.; da Silva, R.M.P.; Araujo, J.V.S.; Milagre, M.X.; de Abreu, C.P.; Machado, C.S.C.; Costa, I. Macro and microgalvanic interactions in friction stir weldment of AA2198-T851 alloy. J. Mater. Res. Technol. 2019, 8, 6209–6222. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, R.M.P.; Izquierdo, J.; Milagre, M.X.; Betancor-Abreu, A.M.; Costa, I.; Souto, R.M. Use of Amperometric and Potentiometric Probes in Scanning Electrochemical Microscopy for the Spatially-Resolved Monitoring of Severe Localized Corrosion Sites on Aluminum Alloy 2098-T351. Sensors 2021, 21, 1132. https://doi.org/10.3390/s21041132
da Silva RMP, Izquierdo J, Milagre MX, Betancor-Abreu AM, Costa I, Souto RM. Use of Amperometric and Potentiometric Probes in Scanning Electrochemical Microscopy for the Spatially-Resolved Monitoring of Severe Localized Corrosion Sites on Aluminum Alloy 2098-T351. Sensors. 2021; 21(4):1132. https://doi.org/10.3390/s21041132
Chicago/Turabian Styleda Silva, Rejane M. P., Javier Izquierdo, Mariana X. Milagre, Abenchara M. Betancor-Abreu, Isolda Costa, and Ricardo M. Souto. 2021. "Use of Amperometric and Potentiometric Probes in Scanning Electrochemical Microscopy for the Spatially-Resolved Monitoring of Severe Localized Corrosion Sites on Aluminum Alloy 2098-T351" Sensors 21, no. 4: 1132. https://doi.org/10.3390/s21041132
APA Styleda Silva, R. M. P., Izquierdo, J., Milagre, M. X., Betancor-Abreu, A. M., Costa, I., & Souto, R. M. (2021). Use of Amperometric and Potentiometric Probes in Scanning Electrochemical Microscopy for the Spatially-Resolved Monitoring of Severe Localized Corrosion Sites on Aluminum Alloy 2098-T351. Sensors, 21(4), 1132. https://doi.org/10.3390/s21041132