PLS-R Calibration Models for Wine Spirit Volatile Phenols Prediction by Near-Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Analytical Procedures
2.2.1. Reagents
2.2.2. Quantification of Volatile Phenols in AWS
2.3. Spectroscopic Measurements
2.4. Data Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schieber, A.; Wüst, M. Volatile Phenols—Important Contributors to the Aroma of Plant-Derived Foods. Molecules 2020, 25, 4529. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Volatile Phenols. In Understanding Wine Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 105–111. [Google Scholar]
- Jeleń, H.H.; Majcher, M.; Szwengiel, A. Key odorants in peated malt whisky and its differentiation from other whisky types using profiling of flavor and volatile compounds. LWT 2019, 107, 56–63. [Google Scholar] [CrossRef]
- Franitza, L.; Granvogl, M.; Schieberle, P. Characterization of the Key Aroma Compounds in Two Commercial Rums by Means of the Sensomics Approach. J. Agric. Food Chem. 2016, 64, 637–645. [Google Scholar] [CrossRef]
- Caldeira, I.; de Sousa, R.B.; Belchior, A.P.; Climaco, M.C. A sensory and chemical approach to the aroma of wooden agend Lourinha wine brandy. Cienc. Tec. Vitiv. 2008, 23, 97–110. [Google Scholar]
- Caldeira, I.; Vitória, C.; Anjos, O.; Fernandes, T.A.; Gallardo, E.; Fargeton, L.; Boissier, B.; Catarino, S.; Canas, S. Wine Spirit Ageing with Chestnut Staves under Different Micro-Oxygenation Strategies: Effects on the Volatile Compounds and Sensory Profile. Appl. Sci. 2021, 11, 3991. [Google Scholar] [CrossRef]
- Granja-Soares, J.; Roque, R.; Cabrita, M.J.; Anjos, O.; Belchior, A.P. Effect of innovative technology using staves and micro-oxygenation on the odorant and sensory pro fi le of aged wine spirit. Food Chem. 2020, 333, 127450. [Google Scholar] [CrossRef]
- Caldeira, I.; Santos, R.; Ricardo-da-Silva, J.M.; Anjos, O.; Mira, H.; Belchior, A.P.; Canas, S. Kinetics of odorant compounds in wine brandies aged in different systems. Food Chem. 2016, 211, 937–946. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Delvaux, F.; Delvaux, F.R. Determination of hydroxycinnamic acids and volatile phenols in wort and beer by isocratic high-performance liquid chromatography using electrochemical detection. J. Chromatogr. A 2006, 1136, 237–242. [Google Scholar] [CrossRef]
- Caldeira, I.; Pereira, R.; Clímaco, M.C.; Belchior, A.P.; de Sousa, R.B. Improved method for extraction of aroma compounds in aged brandies and aqueous alcoholic wood extracts using ultrasound. Anal. Chim. Acta 2004, 513, 125–134. [Google Scholar] [CrossRef]
- Valente, I.M.; Santos, C.M.; Moreira, M.M.; Rodrigues, J.A. New application of the QuEChERS methodology for the determination of volatile phenols in beverages by liquid chromatography. J. Chromatogr. A 2013, 1271, 27–32. [Google Scholar] [CrossRef]
- Castro Mejías, R.; Natera Marín, R.; de Valme García Moreno, M.; García Barroso, C. Optimisation of headspace solid-phase microextraction for the analysis of volatile phenols in wine. J. Chromatogr. A 2003, 995, 11–20. [Google Scholar] [CrossRef]
- Pizarro, C.; Pérez-del-Notario, N.; González-Sáiz, J.M. Optimisation of a simple and reliable method based on headspace solid-phase microextraction for the determination of volatile phenols in beer. J. Chromatogr. A 2010, 1217, 6013–6021. [Google Scholar] [CrossRef]
- Díez, J. Optimisation of stir bar sorptive extraction for the analysis of volatile phenols in wines. J. Chromatogr. A 2004, 1025, 263–267. [Google Scholar] [CrossRef]
- Zhou, Q.; Qian, Y.; Qian, M.C. Analysis of volatile phenols in alcoholic beverage by ethylene glycol-polydimethylsiloxane based stir bar sorptive extraction and gas chromatography–mass spectrometry. J. Chromatogr. A 2015, 1390, 22–27. [Google Scholar] [CrossRef]
- Fariña, L.; Boido, E.; Carrau, F.; Dellacassa, E. Determination of volatile phenols in red wines by dispersive liquid–liquid microextraction and gas chromatography-mass spectrometry detection. J. Chromatogr. A 2007, 1157, 46–50. [Google Scholar] [CrossRef]
- Pizarro, C.; Sáenz-González, C.; Pérez-del-Notario, N.; González-Sáiz, J.M. Ultrasound-assisted emulsification-microextraction for the sensitive determination of Brett character responsible compounds in wines. J. Chromatogr. A 2011, 1218, 8975–8981. [Google Scholar] [CrossRef]
- Downey, G. Tutorial review. Qualitative analysis in the near-infrared region. Analyst 1994, 119, 2367. [Google Scholar] [CrossRef]
- Wang, X. Near-infrared spectroscopy for food quality evaluation. In Evaluation Technologies for Food Quality; Elsevier: Amsterdam, The Netherlands, 2019; pp. 105–118. [Google Scholar]
- Woodcock, T.; Downey, G.; O’Donnell, C.P. Better Quality Food and Beverages: The Role of near Infrared Spectroscopy. J. Near Infrared Spectrosc. 2008, 16, 1–29. [Google Scholar] [CrossRef]
- Lohumi, S.; Lee, S.; Lee, H.; Cho, B.-K. A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration. Trends Food Sci. Technol. 2015, 46, 85–98. [Google Scholar] [CrossRef]
- Cozzolino, D.; Smyth, H.E.; Gishen, M. Feasibility Study on the Use of Visible and Near-Infrared Spectroscopy Together with Chemometrics to Discriminate between Commercial White Wines of Different Varietal Origins. J. Agric. Food Chem. 2003, 51, 7703–7708. [Google Scholar] [CrossRef]
- Da Paixão Teixeira, J.L.; Dos Santos Caramês, E.T.; Baptista, D.P.; Gigante, M.L.; Pallone, J.A.L. Vibrational spectroscopy and chemometrics tools for authenticity and improvement the safety control in goat milk. Food Control 2020, 112, 107105. [Google Scholar] [CrossRef]
- Cozzolino, D.; Smyth, H.E.; Lattey, K.A.; Cynkar, W.; Janik, L.; Dambergs, R.G.; Francis, I.L.; Gishen, M. Relationship between sensory analysis and near infrared spectroscopy in Australian Riesling and Chardonnay wines. Anal. Chim. Acta 2005, 539, 341–348. [Google Scholar] [CrossRef]
- Özdemir, İ.S.; Karaoğlu, Ö.; Dağ, Ç.; Bekiroğlu, S. Assessment of sesame oil fatty acid and sterol composition with FT-NIR spectroscopy and chemometrics. TURKISH J. Agric. For. 2018, 42, 444–452. [Google Scholar] [CrossRef]
- Macedo, L.; Araújo, C.; Vimercati, W.; Hein, P.R.; Pimenta, C.J.; Saraiva, S. Evaluation of chemical properties of intact green coffee beans using near-infrared spectroscopy. J. Sci. Food Agric. 2021, 101, 3500–3507. [Google Scholar] [CrossRef]
- Dhaulaniya, A.S.; Balan, B.; Sodhi, K.K.; Kelly, S.; Cannavan, A.; Singh, D.K. Qualitative and quantitative evaluation of corn syrup as a potential added sweetener in apple fruit juices using mid-infrared spectroscopy assisted chemometric modeling. LWT 2020, 131, 109749. [Google Scholar] [CrossRef]
- Nordon, A.; Mills, A.; Burn, R.T.; Cusick, F.M.; Littlejohn, D. Comparison of non-invasive NIR and Raman spectrometries for determination of alcohol content of spirits. Anal. Chim. Acta 2005, 548, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Mendes, L.S.; Oliveira, F.C.C.; Suarez, P.A.Z.; Rubim, J.C. Determination of ethanol in fuel ethanol and beverages by Fourier transform (FT)-near infrared and FT-Raman spectrometries. Anal. Chim. Acta 2003, 493, 219–231. [Google Scholar] [CrossRef]
- Palma, M. Application of FT-IR spectroscopy to the characterisation and classification of wines, brandies and other distilled drinks. Talanta 2002, 58, 265–271. [Google Scholar] [CrossRef]
- Kolomiets, O.A.; Lachenmeier, D.W.; Hoffmann, U.; Siesler, H.W. Quantitative Determination of Quality Parameters and Authentication of Vodka Using near Infrared Spectroscopy. J. Near Infrared Spectrosc. 2010, 18, 59–67. [Google Scholar] [CrossRef]
- Yang, Y.R.; Ren, Y.F.; Dong, G.M.; Yang, R.J.; Liu, H.X.; Du, Y.H.; Zhang, W.Y. Determination of Methanol in Alcoholic Beverages by Two-Dimensional Near-Infrared Correlation Spectroscopy. Anal. Lett. 2016, 49, 2279–2289. [Google Scholar] [CrossRef]
- Mosedale, J.; Puech, J. Wood maturation of distilled beverages. Trends Food Sci. Technol. 1998, 9, 95–101. [Google Scholar] [CrossRef]
- França, L.; Grassi, S.; Pimentel, M.F.; Amigo, J.M. A single model to monitor multistep craft beer manufacturing using near infrared spectroscopy and chemometrics. Food Bioprod. Process. 2021, 126, 95–103. [Google Scholar] [CrossRef]
- Power, A.C.; Jones, J.; NiNeil, C.; Geoghegan, S.; Warren, S.; Currivan, S.; Cozzolino, D. What’s in this drink? Classification and adulterant detection in Irish Whiskey samples using near infrared spectroscopy combined with chemometrics. J. Sci. Food Agric. 2021, 101, 5256–5263. [Google Scholar] [CrossRef]
- Hanousek-Cica, K.; Pezer, M.; Mrvcic, J.; Stanzer, D.; Cacic, J.; Jurak, V.; Krajnovic, M.; Gajdos-Kljusuric, J. Identification of phenolic and alcoholic compounds in wine spirits and their classification by use of multivariate analysis. J. Serb. Chem. Soc. 2019, 84, 663–677. [Google Scholar] [CrossRef] [Green Version]
- Anjos, O.; Caldeira, I.; Roque, R.; Pedro, S.I.; Lourenço, S. Screening of Different Ageing Technologies of Wine Spectroscopy and Volatile Quantification. Processes 2020, 8, 8–18. [Google Scholar]
- Anjos, O.; Santos, A.J.A.; Estevinho, L.M.; Caldeira, I. FTIR–ATR spectroscopy applied to quality control of grape-derived spirits. Food Chem. 2016, 205, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Canas, S.; Caldeira, I.; Anjos, O.; Belchior, A.P. Phenolic profile and colour acquired by the wine spirit in the beginning of ageing: Alternative technology using micro-oxygenation vs traditional technology. LWT 2019, 111, 260–269. [Google Scholar] [CrossRef]
- Jakubíková, M.; Sádecká, J.; Kleinová, A.; Májek, P. Near-infrared spectroscopy for rapid classification of fruit spirits. J. Food Sci. Technol. 2016, 53, 2797–2803. [Google Scholar] [CrossRef] [Green Version]
- Schiavone, S.; Marchionni, B.; Bucci, R.; Marini, F.; Biancolillo, A. Authentication of Grappa (Italian grape marc spirit) by Mid and Near Infrared spectroscopies coupled with chemometrics. Vib. Spectrosc. 2020, 107, 103040. [Google Scholar] [CrossRef]
- Giannetti, V.; Mariani, M.B.; Marini, F.; Torrelli, P.; Biancolillo, A. Grappa and Italian spirits: Multi-platform investigation based on GC–MS, MIR and NIR spectroscopies for the authentication of the Geographical Indication. Microchem. J. 2020, 157, 104896. [Google Scholar] [CrossRef]
- Li, S.; Shan, Y.; Zhu, X.; Zhang, X.; Ling, G. Detection of honey adulteration by high fructose corn syrup and maltose syrup using Raman spectroscopy. J. Food Compos. Anal. 2012, 28, 69–74. [Google Scholar] [CrossRef]
- Chen, H.; Tan, C.; Wu, T.; Wang, L.; Zhu, W. Discrimination between authentic and adulterated liquors by near-infrared spectroscopy and ensemble classification. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 245–249. [Google Scholar] [CrossRef]
- Schwarz, M.; Rodríguez, M.; Martínez, C.; Bosquet, V.; Guillén, D.; Barroso, C.G. Antioxidant activity of Brandy de Jerez and other aged distillates, and correlation with their polyphenolic content. Food Chem. 2009, 116, 29–33. [Google Scholar] [CrossRef]
- Tsakiris, A.; Kallithraka, S.; Kourkoutas, Y. Grape brandy production, composition and sensory evaluation. J. Sci. Food Agric. 2014, 94, 404–414. [Google Scholar] [CrossRef]
- Workman, J., Jr.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy; Taylor & Francis Group: Boca Raton, FL, USA, 2007; ISBN 978-1-57444-784-2. [Google Scholar]
- Yu, H.; Zhou, Y.; Fu, X.; Xie, L.; Ying, Y. Discrimination between Chinese rice wines of different geographical origins by NIRS and AAS. Eur. Food Res. Technol. 2007, 225, 313–320. [Google Scholar] [CrossRef]
- Cozzolino, D.; Corbella, E. Determination of honey quality components by near infrared reflectance spectroscopy. J. Apic. Res. 2003, 42, 16–20. [Google Scholar] [CrossRef]
- Pardeshi, S.; Dhodapkar, R.; Kumar, A. Quantum chemical density functional theory studies on the molecular structure and vibrational spectra of Gallic acid imprinted polymers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 562–573. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Williams, P.; Dardenne, P.; Flinn, P. Tutorial: Items to be included in a report on a near infrared spectroscopy project. J. Near Infrared Spectrosc. 2017, 25, 85–90. [Google Scholar] [CrossRef]
- Conzen, J.P. Multivariate Calibration. A Practical Guide for the Method Development in the Analytical Chemistry, 2nd ed.; Bruker Optick: Ettlingen, Germany, 2006. [Google Scholar]
- Dambergs, R.G.; Kambouris, A.; Schumacher, N.; Francis, I.L.; Esler, M.B.; Gishen, M. Wine quality grading by near infrared spectroscopy. In Near Infrared Spectroscopy: Proceedings of the 10th International Conference; IMPublications Open: Chichester, UK, 2001; pp. 187–190. ISBN 978-1-906715-22-9. [Google Scholar]
- De Carvalho, L.C.; de Lelis Medeiros de Morais, C.; de Lima, K.M.G.; Cunha, L.C., Jr.; Martins Nascimento, P.A.; Bosco de Faria, J.; de Almeida Teixeira, G.H. Determination of the geographical origin and ethanol content of Brazilian sugarcane spirit using near-infrared spectroscopy coupled with discriminant analysis. Anal. Methods 2016, 8, 5658–5666. [Google Scholar] [CrossRef]





| Chestnut Wood (C) | Oak Wood (L) | Total | ||||
|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | |||
| (B) 250 L wooden barrel | 6 * | 6 * | 6 * | 6 * | 24 | |
| 50 L glass demijohns with wood staves with MOX | (15) with a flow rate of 2 mL/L/month during the first 15 days followed by 0.6 mL/L/month until 365 days | 6 * | 6 * | 6 * | 6 * | 24 |
| (30) flow rate of 2 mL/L/month during the first 30 days followed by 0.6 mL/L/month until 365 days | 6 * | 6 * | 6 * | 6 * | 24 | |
| (60) a flow rate of 2 mL/L/month during the first 60 days followed by 0.6 mL/L/month until 365 days | 6 * | 6 * | 6 * | 6 * | 24 | |
| (N) nitrogen application with a flow rate of 20 mL/L/month | 6 * | 6 * | 6 * | 6 * | 24 | |
| Total | 30 | 30 | 30 | 30 | 120 | |
| Volatile Phenol | Number of Samples | N | Mean ± SD | Min–Max | CV (%) | LOQ 1 |
|---|---|---|---|---|---|---|
| Guaiacol (mg/L) | Set1 | 56 | 0.491 ± 0.165 | 0.098–0.696 | 33.65 | 0.037 |
| Set2 | 56 | 0.489 ± 0.158 | 0.095–0.699 | 32.31 | ||
| Set1 + Set2 | 112 | 0.487 ± 0.158 | 0.095–0.696 | 32.33 | ||
| 4-methyl-guaiacol (mg/L) | Set1 | 56 | 0.279 ± 0.109 | 0.073–0.487 | 39.07 | 0.033 |
| Set2 | 56 | 0.280 ± 0.101 | 0.073–0.478 | 38.92 | ||
| Set1 + Set2 | 112 | 0.279 ± 0.174 | 0.073–0.487 | 37.75 | ||
| Eugenol (mg/L) | Set1 | 54 | 0.291 ± 0.020 | 0.252–0.350 | 6.91 | 0.021 |
| Set2 | 54 | 0.290 ± 0.019 | 0.251–0.328 | 6.57 | ||
| Set1 + Set2 | 108 | 0.289 ± 0.021 | 0.252–0.328 | 7.22 | ||
| Syringol (mg/L) | Set1 | 54 | 1.708 ± 0.705 | 0.221–3.172 | 41.31 | 0.029 |
| Set2 | 54 | 1.679 ± 0.683 | 0.244–3.106 | 40.66 | ||
| Set1 + Set2 | 108 | 1.702 ± 0.695 | 0.221–3.172 | 39.65 | ||
| 4-methyl-syringol (mg/L) | Set1 | 55 | 1.034 ± 0.383 | 0.274–1.552 | 37.04 | 0.034 |
| Set2 | 55 | 1.090 ± 0.395 | 0.259–1.536 | 36.21 | ||
| Set1 + Set2 | 110 | 1.043 ± 0.393 | 0.259–1.552 | 37.66 | ||
| 4-allyl-syringol (mg/L) | Set1 | 51 | 0.414 ± 0.076 | 0.273–0.55 | 18.32 | 0.043 |
| Set2 | 51 | 0.416 ± 0.078 | 0.255–0.578 | 18.80 | ||
| Set1 + Set2 | 102 | 0.417 ± 0.075 | 0.255–0.578 | 17.87 |
| Volatile Phenol | Spectral Range (cm−1) | Pre-Process | Rk | r2 | RMSEP | RMSECV | RMSEC | RPD | Bias | |
|---|---|---|---|---|---|---|---|---|---|---|
| Guaiacol | 9118.1–5415.3 | 1stDer + MSC | Set 1 | 10 | 96.80 | 0.0296 | 5.90 | −0.0095 | ||
| Set 2 | 5 | 96.84 | 0.0270 | 5.63 | 0.0004 | |||||
| Set 1 + 2 | 8 | 96.34 | 0.0298 | 5.23 | ||||||
| 4-methyl-guaiacol | 8304.2–7347.7 6869.4–5434.6 4956.3–4478 | 1stDer + SLS | Set 1 | 10 | 96.34 | 0.0233 | 5.36 | −0.0052 | ||
| Set 2 | 10 | 92.70 | 0.0204 | 3.7 | 0.0006 | |||||
| Set 1 + 2 | 10 | 96.10 | 0.0218 | 5.07 | ||||||
| Eugenol | 9337.9–5446.2 | 1stDer + SLS | Set 1 | 7 | 95.30 | 0.0049 | 4.92 | −0.0017 | ||
| Set 2 | 10 | 92.30 | 0.0053 | 3.59 | 0.0001 | |||||
| Set 1 + 2 | 10 | 96.06 | 0.0044 | 5.04 | ||||||
| Syringol | 6101.9–5446.2 | 1stDer + SLS | Set 1 | 9 | 97.81 | 0.1170 | 6.76 | −0.0028 | ||
| Set 2 | 8 | 93.74 | 0.1560 | 4.50 | −0.0028 | |||||
| Set 1 + 2 | 10 | 97.32 | 0.1170 | 6.11 | ||||||
| 4-methyl-syringol | 9160.5–4512.7 | 1stDer + SLS | Set 1 | 10 | 94.88 | 0.0874 | 4.45 | −0.0108 | ||
| Set 2 | 10 | 90.42 | 0.0653 | 3.23 | −0.0024 | |||||
| Set 1 + 2 | 10 | 95.79 | 0.0772 | 4.88 | ||||||
| 4-allyl-syringol | 9353.3–7498.1 6101.9–5446.2 | 1stDer + MSC | Set 1 | 8 | 90.05 | 0.0176 | 3.19 | −0.0018 | ||
| Set 2 | 10 | 92.44 | 0.0243 | 3.64 | −0.0011 | |||||
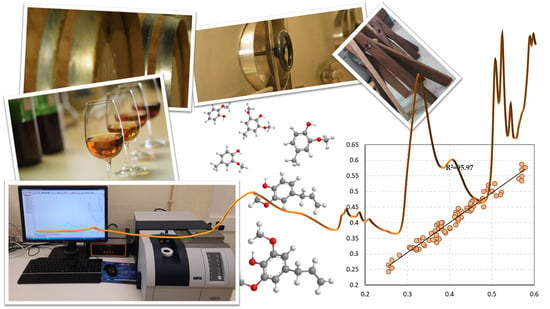
| Set 1 + 2 | 10 | 95.97 | 0.0159 | 4.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjos, O.; Caldeira, I.; Fernandes, T.A.; Pedro, S.I.; Vitória, C.; Oliveira-Alves, S.; Catarino, S.; Canas, S. PLS-R Calibration Models for Wine Spirit Volatile Phenols Prediction by Near-Infrared Spectroscopy. Sensors 2022, 22, 286. https://doi.org/10.3390/s22010286
Anjos O, Caldeira I, Fernandes TA, Pedro SI, Vitória C, Oliveira-Alves S, Catarino S, Canas S. PLS-R Calibration Models for Wine Spirit Volatile Phenols Prediction by Near-Infrared Spectroscopy. Sensors. 2022; 22(1):286. https://doi.org/10.3390/s22010286
Chicago/Turabian StyleAnjos, Ofélia, Ilda Caldeira, Tiago A. Fernandes, Soraia Inês Pedro, Cláudia Vitória, Sheila Oliveira-Alves, Sofia Catarino, and Sara Canas. 2022. "PLS-R Calibration Models for Wine Spirit Volatile Phenols Prediction by Near-Infrared Spectroscopy" Sensors 22, no. 1: 286. https://doi.org/10.3390/s22010286
APA StyleAnjos, O., Caldeira, I., Fernandes, T. A., Pedro, S. I., Vitória, C., Oliveira-Alves, S., Catarino, S., & Canas, S. (2022). PLS-R Calibration Models for Wine Spirit Volatile Phenols Prediction by Near-Infrared Spectroscopy. Sensors, 22(1), 286. https://doi.org/10.3390/s22010286