Application of Digital Olfaction for Table Olive Industry
Abstract
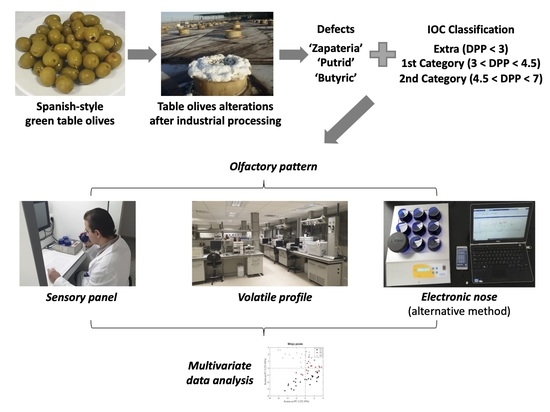
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Analyses
2.2.1. Sensory Analysis
2.2.2. Analysis of Volatile Compounds
2.2.3. E-Nose Analysis
2.2.4. Multivariate Data Analysis
3. Results
3.1. Sensory Analysis of Spanish-Style Table Olives
3.2. Volatile Profile of Spanish-Style Table Olives
3.3. E-Nose Classification of Spanish-Style Table Olives
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministerio de Agricultura pesca y Alimentación Aceituna de Mesa. Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/aceite-oliva-y-aceituna-mesa/aceituna.aspx (accessed on 9 May 2022).
- Royal Degree 679/2016, Norma de Calidad de Las Aceitunas de Mesa. Boletín Oficial del Estado. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2016-11953 (accessed on 17 May 2022).
- IOC. Method for the Sensory Analysis of Table Olives COI/OT/MO/Doc. No 1/Rev.3; IOC: Lausanne, Switzerland, 2021. [Google Scholar]
- Lanza, B.; Amoruso, F. Sensory Analysis of Natural Table Olives: Relationship between Appearance of Defect and Gustatory-Kinaesthetic Sensation Changes. LWT Food Sci. Technol. 2016, 68, 365–372. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Schaide, T.; Boselli, E.; Martínez, M.; Arias-Calderón, R.; Pérez-Nevado, F. Effects of Different Controlled Temperatures on Spanish-Style Fermentation Processes of Olives. Foods 2021, 10, 666. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Cabrera-Bañegil, M.; Fernández, A.; Delgado-Adámez, J.; Ramírez, R.; Martín-Vertedor, D. Monitoring of Acrylamide and Phenolic Compounds in Table Olive after High Hydrostatic Pressure and Cooking Treatments. Food Chem. 2019, 286, 250–259. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Sánchez-Gómez, A.H.; Montaño, A.; Cortés-Delgado, A.; Garrido-Fernández, A. Sensory Characterisation of Black Ripe Table Olives from Spanish Manzanilla and Hojiblanca Cultivars. Food Res. Int. 2019, 116, 114–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panagou, E.Z.; Sahgal, N.; Magan, N.; Nychas, G.J.E. Table Olives Volatile Fingerprints: Potential of an Electronic Nose for Quality Discrimination. Sens. Actuators B Chem. 2008, 134, 902–907. [Google Scholar] [CrossRef]
- Xu, J.; Liu, K.; Zhang, C. Electronic Nose for Volatile Organic Compounds Analysis in Rice Aging. Trends Food Sci. Technol. 2021, 109, 83–93. [Google Scholar] [CrossRef]
- Martínez-García, R.; Moreno, J.; Bellincontro, A.; Centioni, L.; Puig-Pujol, A.; Peinado, R.A.; Mauricio, J.C.; García-Martínez, T. Using an Electronic Nose and Volatilome Analysis to Differentiate Sparkling Wines Obtained under Different Conditions of Temperature, Ageing Time and Yeast Formats. Food Chem. 2021, 334, 127574. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Wang, Y.; Kong, B.; Chen, Q. Evaluation of the Flavour Properties of Cooked Chicken Drumsticks as Affected by Sugar Smoking Times Using an Electronic Nose, Electronic Tongue, and HS-SPME/GC-MS. LWT 2021, 140, 110764. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic Noses in Classification and Quality Control of Edible Oils: A Review. Food Chem. 2018, 246, 192–201. [Google Scholar] [CrossRef]
- Arroyo, P.; Meléndez, F.; Suárez, J.I.; Herrero, J.L.; Rodríguez, S.; Lozano, J. Electronic Nose with Digital Gas Sensors Connected via Bluetooth to a Smartphone for Air Quality Measurements. Sensors 2020, 20, 786. [Google Scholar] [CrossRef] [Green Version]
- Martínez Gila, D.M.; Gámez García, J.; Bellincontro, A.; Mencarelli, F.; Gómez Ortega, J. Fast Tool Based on Electronic Nose to Predict Olive Fruit Quality after Harvest. Postharvest Biol. Technol. 2020, 160, 111058. [Google Scholar] [CrossRef]
- Schaide, T.; Cabrera-Bañegil, M.; Pérez-Nevado, F.; Esperilla, A.; Martín-Vertedor, D. Effect of Olive Leaf Extract Combined with Saccharomyces Cerevisiae in the Fermentation Process of Table Olives. J. Food Sci. Technol. 2019, 56, 3001–3013. [Google Scholar] [CrossRef]
- Portalo-Calero, F.; Arroyo, P.; Suárez, J.I.; Lozano, J. Triangular Test of Amanita Mushrooms by Using Electronic Nose and Sensory Panel. Foods 2019, 8, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arroyo, P.; Lozano, J.; Suárez, J.I. Evolution of Wireless Sensor Network for Air Quality Measurements. Electronics 2018, 7, 342. [Google Scholar] [CrossRef] [Green Version]
- Barker, M.; Rayens, W. Partial Least Squares for Discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Lanza, B. Abnormal fermentations in table-olive processing: Microbial origin and sensory evaluation. Front. Microbiol. 2013, 4, 91. [Google Scholar] [CrossRef] [Green Version]
- Marx, Í.M.G.; Rodrigues, N.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Drunkler, D.A.; Peres, A.M. Quantification of Table Olives’ Acid, Bitter and Salty Tastes Using Potentiometric Electronic Tongue Fingerprints. LWT Food Sci. Technol. 2017, 79, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Mas, A.; Guillamon, J.M.; Torija, M.J.; Beltran, G.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Bioactive Compounds Derived from the Yeast Metabolism of Aromatic Amino Acids during Alcoholic Fermentation. BioMed Res. Int. 2014, 2014, 898045. [Google Scholar] [CrossRef]
- Fischer, T.; Pietruszka, J. Key Building Blocks via Enzyme-Mediated Synthesis. Top. Curr. Chem. 2010, 297, 1–43. [Google Scholar] [CrossRef]
- Wang, B.; Sun, M.; Yang, J.; Shen, Z.; Ou, Y.; Fu, L.; Zhao, Y.; Li, R.; Ruan, Y.; Shen, Q. Inducing Banana Fusarium Wilt Disease Suppression through Soil Microbiome Reshaping by Pineapple-Banana Rotation Combined with Biofertilizer Application. Soil 2022, 8, 17–29. [Google Scholar] [CrossRef]
- Montaño, A.; de Castro, A.; Rejano, L.; Sánchez, A.H. Analysis of Zapatera Olives by Gas and High-Performance Liquid Chromatography. J. Chromatogr. A 1992, 594, 259–267. [Google Scholar] [CrossRef]
- de Castro, A.; Sánchez, A.H.; López-López, A.; Cortés-Delgado, A.; Medina, E.; Montaño, A. Microbiota and Metabolite Profiling of Spoiled Spanish-Style Green Table Olives. Metabolites 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansone-Land, A.; Takeoka, G.R.; Shoemaker, C.F. Volatile Constituents of Commercial Imported and Domestic Black-Ripe Table Olives (Olea europaea). Food Chem. 2014, 149, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhang, H.; Liu, T.; Gong, P.; Wang, Y.; Wang, H.; Tian, X.; Liu, Q.; Cui, Q.; Xie, X.; et al. Aroma Classification and Flavor Characterization of Streptococcus Thermophilus Fermented Milk by HS-GC-IMS and HS-SPME-GC-TOF/MS. Food Biosci. 2022, 49, 101832. [Google Scholar] [CrossRef]
- Sánchez, R.; Martín-tornero, E.; Lozano, J.; Boselli, E.; Arroyo, P.; Meléndez, F.; Martín-vertedor, D. E-nose Discrimination of Abnormal Fermentations in Spanish-style Green Olives. Molecules 2021, 26, 5353. [Google Scholar] [CrossRef]
- Sánchez, R.; Pérez-Nevado, F.; Montero-Fernández, I.; Lozano, J.; Meléndez, F.; Martín-Vertedor, D. Application of Electronic Nose to Discriminate Species of Mold Strains in Synthetic Brines. Front. Microbiol. 2022, 13, 897178. [Google Scholar] [CrossRef] [PubMed]
- Mota, I.; Teixeira-Santos, R.; Cavaleiro Rufo, J. Detection and Identification of Fungal Species by Electronic Nose Technology: A Systematic Review. Fungal Biol. Rev. 2021, 37, 59–70. [Google Scholar] [CrossRef]
- Loulier, J.; Lefort, F.; Stocki, M.; Asztemborska, M.; Szmigielski, R.; Siwek, K.; Grzywacz, T.; Hsiang, T.; Ślusarski, S.; Oszako, T.; et al. Detection of Fungi and Oomycetes by Volatiles Using E-Nose and Spme-Gc/Ms Platforms. Molecules 2020, 25, 5749. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Fernández, A.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. Electronic nose application for the discrimination of sterilization treatments applied to Californian-style black olive varieties. J. Sci. Food Agric. 2022, 102, 2232–2241. [Google Scholar] [CrossRef]
- Sánchez, R.; Martín-Tornero, E.; Lozano, J.; Arroyo, P.; Meléndez, F.; Martín-Vertedor, D. Evaluation of the olfactory pattern of black olives stuffed with flavored hydrocolloids. LWT 2022, 163, 113556. [Google Scholar] [CrossRef]
- Sánchez, R.; Boselli, E.; Fernández, A.; Arroyo, P.; Lozano, J.; Martín-Vertedor, D. Determination of the Masking Effect of the ‘Zapateria’Defect in Flavoured Stuffed Olives Using E-Nose. Molecules 2022, 27, 4300. [Google Scholar] [CrossRef] [PubMed]




| Extra | 1st Category | 2nd Category | ||||
|---|---|---|---|---|---|---|
| DPP ≤ 3 | 3 < DPP ≤ 4.5 | 4.5 < DPP ≤ 7.0 | ||||
| Sensory evaluation | ‘No defect’ | ‘Zapateria’ | ‘Putrid’ | ‘Butyric’ | ‘Zapateria’ | ‘Putrid’ |
| n.d. n = 18 | 4.1 ± 0.9 ns A n = 15 | 3.8 ± 0.7 ns A n = 15 | 3.9 ± 0.8 ns n = 15 | 6.2 ± 0.8 ns B n = 9 | 5.9 ± 0.9 ns B n = 9 | |
| RT (min) | Volatile Compounds | Extra | 1st. Category | 2nd. Category | |||
|---|---|---|---|---|---|---|---|
| ‘Zapateria’ | ‘Putrid’ | ‘Butyric’ | ‘Zapateria’ | ‘Putrid’ | |||
| 1.8 | Isopropyl alcohol | 17.2 ± 1.3 * | 21.3 ± 4.2 * | ||||
| 2.4 | Butan-2-ol | 4.5 ± 0.6 * | |||||
| 2.7 | Acetic acid | 9.6 ± 1.5 | 8.3 ± 1.2 | 5.4 ± 0.7 | 7.5 ± 1.2 | 1.9 ± 0.5 | |
| 4.8 | 2-methyl-butan-1-ol | 5.2 ± 0.8 | 6.5 ± 2.2 | ||||
| 4.9 | Propanoic acid | 0.9 ± 0.2 | 17.2 ± 7.4 | 3.6 ± 0.8 | 2.6 ± 0.6 | 23.4 ± 8.7 | |
| 5.8 | Propylene glycol | 9.6 ± 1.1 * | 15.9 ± 5.8 * | ||||
| 6.7 | 2,4-dimethyl-heptane | 1.3 ± 0.5 | 15.4 ± 5.7 | 17.9 ± 8.6 | |||
| 8.2 | Butanoic acid | 14.6 ± 4.6 | 40.7 ± 8.4 | 22.8 ± 9.5 | |||
| 9.7 | (Z)-3-Hexen-1-ol | 0.7 ± 0.1 * | |||||
| 11.2 | Styrene | ||||||
| 13.5 | Pentanoic acid | 3.2 ± 0.6 | 11.8 ± 2.7 | 4.5 ± 2.4 | |||
| 17.8 | 2,4-Hexadienoic acid, methyl ester | 3.2 ± 0.7 * | 9.6 ± 3.7 * | ||||
| 18.5 | Hexanoic acid | 1.8 ± 0.6 * | 5.5 ± 1.1 * | ||||
| 20.7 | (E)-3-Hexenoic acid | 8.2 ± 1.2 * | 14.4 ± 6.7 * | ||||
| 21.9 | 2-methoxy-phenol | 4.0 ± 0.4 | 2.6 ± 0.7 | 0.8 ± 0.2 | 4.1 ± 0.6 | ||
| 22.0 | 2,4-Hexadienoic acid, ethyl ester | 3.1 ± 0.6 * | 9.5 ± 2.1 * | ||||
| 23.0 | 2-Ethenyl-1,1-dimethyl-3-methylene-cyclohexane | 18.0 ± 2.6 * | |||||
| 23.3 | Phenylethyl Alcohol | 9.7 ± 1.5 | 12.0 ± 4,4 | 19.6 ± 8.9 | |||
| 26.5 | Cyclohexanecarboxylic acid | 1.8 ± 0.4 * | 7.4 ± 2.1 * | ||||
| 27.0 | Creosol | 48.1 ± 6.8 | 35.2 ± 9.4 | 27.5 ± 9.6 | 25.4 ± 10.4 | 3.2 ± 0.5 | 7.2 ± 0.8 |
| 28.2 | Benzoic acid | 8.6 ± 1.3 | 7.5 ± 5.9 | 6.5 ± 3.6 | 1.9 ± 0.4 | ||
| Predicted Class | |||
|---|---|---|---|
| Real Class | Extra | 1st Category | 2nd Category |
| Extra | 18 | 0 | 0 |
| 1st Category | 0 | 45 | 0 |
| 2nd Category | 0 | 0 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, R.; Fernández, A.; Martín-Tornero, E.; Meléndez, F.; Lozano, J.; Martín-Vertedor, D. Application of Digital Olfaction for Table Olive Industry. Sensors 2022, 22, 5702. https://doi.org/10.3390/s22155702
Sánchez R, Fernández A, Martín-Tornero E, Meléndez F, Lozano J, Martín-Vertedor D. Application of Digital Olfaction for Table Olive Industry. Sensors. 2022; 22(15):5702. https://doi.org/10.3390/s22155702
Chicago/Turabian StyleSánchez, Ramiro, Antonio Fernández, Elisabet Martín-Tornero, Félix Meléndez, Jesús Lozano, and Daniel Martín-Vertedor. 2022. "Application of Digital Olfaction for Table Olive Industry" Sensors 22, no. 15: 5702. https://doi.org/10.3390/s22155702
APA StyleSánchez, R., Fernández, A., Martín-Tornero, E., Meléndez, F., Lozano, J., & Martín-Vertedor, D. (2022). Application of Digital Olfaction for Table Olive Industry. Sensors, 22(15), 5702. https://doi.org/10.3390/s22155702