AC-Electroosmosis-Assisted Surface Plasmon Resonance Sensing for Enhancing Protein Signals with a Simple Kretschmann Configuration
Abstract
:1. Introduction
2. Materials and Methods
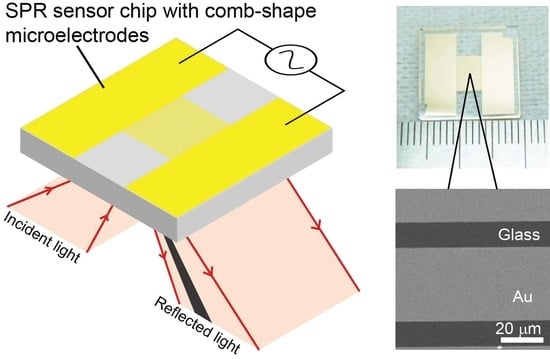
2.1. Sensor Chip Fabrication
2.2. Sensing Operation
2.3. ACEO Evaluation
2.4. Protein Detaction
3. Results and Discussion
3.1. ACEO on the Sensor Chip
3.2. Enhancement of Protein Binding
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homola, J.; Gauglitz, G. Surface plasmon resonance sensors. Sens. Actuators B 1999, 54, 3–5. [Google Scholar] [CrossRef]
- Gervais, L.; de Rooij, N.; Delamarche, E. Microfluidic chips for point-of-care immunodiagnostics. Adv. Mater. 2011, 23, H151–H176. [Google Scholar] [CrossRef]
- Hoa, X.D.; Kirk, A.G.; Tabrizian, M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens. Bioelectron. 2007, 23, 151–160. [Google Scholar] [CrossRef]
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Ince, R.; Narayanaswamy, R. Analysis of the performance of interferometry, surface plasmon resonance and luminescence as biosensors and chemosensors. Anal. Chim. Acta 2006, 569, 1–20. [Google Scholar] [CrossRef]
- Kurihara, K.; Suzuki, K. Theoretical understanding of an absorption-based surface plasmon resonance sensor based on Kretchmann’s theory. Anal. Chem. 2002, 74, 696–701. [Google Scholar] [CrossRef]
- Rich, R.L.; Myszka, D.G. Survey of the year 2006 commercial optical biosensor literature. J. Mol. Recognit. 2007, 20, 300–366. [Google Scholar] [CrossRef]
- Nagase, N.; Terao, K.; Miyanishi, N.; Tamai, K.; Uchiyama, N.; Suzuki, T.; Takao, H.; Shimokawa, F.; Oohira, F. Signal enhancement of protein binding by electrodeposited gold nanostructures for applications in Kretschmann-type SPR sensors. Analyst 2012, 137, 5034–5040. [Google Scholar] [CrossRef]
- Terao, K.; Hiramatsu, S.; Suzuki, T.; Takao, H.; Shimokawa, F.; Oohira, F. Fast protein detection in raw blood by size-exclusion SPR sensing. Anal. Methods 2015, 7, 6483–6488. [Google Scholar] [CrossRef]
- Terao, K.; Shimizu, K.; Miyanishi, N.; Shimamoto, S.; Suzuki, T.; Takao, H.; Oohira, F. Size-exclusion SPR sensor chip: Application to detection of aggregation and disaggregation of biological particles. Analyst 2012, 137, 2192–2198. [Google Scholar] [CrossRef]
- Brown, M.R.; Meinhart, C.D. AC electroosmotic flow in a DNA concentrator. Microfluid Nanofluid 2006, 2, 513–523. [Google Scholar]
- Ramos, A.; Morgan, H.; Green, N.G.; Castellanos, A. AC electric-field-induced fluid flow in microelectrodes. J. Colloid Interface Sci. 1999, 217, 420–422. [Google Scholar] [CrossRef]
- Selmi, M.; Belmabrouk, H. AC electroosmosis effect on microfluidic heterogeneous immunoassay efficiency. Micromachines 2020, 11, 342. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Chen, P.; Chung, M.T.; Nidetz, R.; Park, Y.; Liu, Z.; McHugh, W.; Cornell, T.T.; Fu, J.; Kurabayashi, K. AC electroosmosis-enhanced nanoplasmofluidic detection of ultralow-concentration cytokine. Nano Lett. 2017, 17, 2374–2380. [Google Scholar] [CrossRef] [Green Version]
- Shiddiky, M.J.A.; Vaidyanathan, R.; Rauf, S.; Tay, Z.; Trau, M. Molecular nanoshearing: An innovative approach to shear off molecules with AC-induced nanoscopic fluid flow. Sci. Rep. 2014, 4, 3716. [Google Scholar] [CrossRef] [Green Version]
- Avenas, Q.; Moreau, J.; Costella, M.; Maalaoui, A.; Souifi, A.; Charette, P.; Marchalot, J.; Robin, M.F.; Canva, M. Performance improvement of plasmonic sensors using a combination of AC electrokinetic effects for (bio)target capture. Electrophoresis 2019, 40, 1426–1435. [Google Scholar] [CrossRef]
- Costella, M.; Robin, M.F.; Marchalot, J.; Moreau, J.; Andreiev, O.; Canva, M.; Charette, P. Surface plasmon resonance imaging enhanced by dielectrophoresis and AC-electroosmosis. Proc. SPIE 2020, 11257, 1125703. [Google Scholar]
- Campbell, C.T.; Kim, G. SPR microscopy and its applications to high-throughput analyses of biomolecular binding events and their kinetics. Biomaterials 2007, 28, 2380–2392. [Google Scholar] [CrossRef]
- Bardin, F.; Bellemain, A.; Roger, G.; Canva, M. Surface plasmon resonance spectro-imaging sensor for biomolecular surface interaction characterization. Biosens. Bioelectron. 2009, 24, 2100–2105. [Google Scholar] [CrossRef]
- Lioubimov, V.; Kolomenskii, A.; Mershin, A.; Nanopoulos, D.V.; Schuessler, H.A. Effect of varying electric potential on surface-plasmon resonance sensing. Appl. Optics 2004, 43, 3426–3432. [Google Scholar] [CrossRef]
- Foley, K.J.; Shan, X.; Tao, N.J. Surface impedance imaging technique. Anal. Chem. 2008, 80, 5146–5151. [Google Scholar] [CrossRef]
- González, A.; Ramos, A.; Green, N.G.; Castellanos, A.; Morgan, H. Fluid flow induced by nonuniform ac electric fields in electrolytes on microelectrodes. II. A linear double-layer analysis. Phys. Rev. E 2000, 61, 4019. [Google Scholar] [CrossRef] [Green Version]
- Uludag, Y.; Tothill, I.E. Cancer biomarker detection in serum samples using surface plasmon resonance and quartz crystal microbalance sensors with nanoparticle signal amplification. Anal. Chem. 2012, 84, 5898–5904. [Google Scholar] [CrossRef]
- Sakai, G.; Nakata, S.; Uda, T.; Miura, N.; Yamazoe, N. Highly selective and sensitive SPR immunosensor for detection of methamphetamine. Electrochim. Acta 1999, 44, 3849–3854. [Google Scholar] [CrossRef]
- Treviño, J.; Calle, A.; Rodríguez-Frade, J.M.; Mellado, M.; Lechuga, L.M. Surface plasmon resonance immunoassay analysis of pituitary hormones in urine and serum samples. Clin. Chim. Acta 2009, 403, 56–62. [Google Scholar] [CrossRef]
- Stevens, R.C.; Soelberg, S.D.; Near, S.; Furlong, C.E. Detection of cortisol in saliva with a flow-filtered, portable surface plasmon resonance biosensor system. Anal. Chem. 2008, 80, 6747–6751. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.S.; Lowe, T.E.; Ingram, J.R. Rapid ultrasensitive measurement of salivary cortisol using nano-linker chemistry coupled with surface plasmon resonance detection. Analyst 2009, 134, 380–386. [Google Scholar] [CrossRef]
- Frasconi, M.; Mazzarino, M.; Botrè, F.; Mazzei, F. Surface plasmon resonance immunosensor for cortisol and cortisone determination. Anal. Bioanal. Chem. 2009, 394, 2151–2159. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Carlen, E.T.; Bomer, J.G.; Wijinperlé, D.; DeBoer, H.L.; van den Berg, A.; Schasfoort, R.B.M. Electrokinetic label-free screening chip: A marriage of multiplexing and high throughput analysis using surface plasmon resonance imaging. Lab Chip 2010, 10, 986–990. [Google Scholar] [CrossRef]
- Hsieh, H.; Luo, J.; Shen, Y.; Lo, S.; Hsu, Y.; Tahara, H.; Fan, Y.; Wei, P.; Sheen, H. A nanofluidic preconcentrator integrated with an aluminum-based nanoplasmonic sensor for Epstein-Barr virus detection. Sens. Actuators B 2022, 355, 131327. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terao, K.; Kondo, S. AC-Electroosmosis-Assisted Surface Plasmon Resonance Sensing for Enhancing Protein Signals with a Simple Kretschmann Configuration. Sensors 2022, 22, 854. https://doi.org/10.3390/s22030854
Terao K, Kondo S. AC-Electroosmosis-Assisted Surface Plasmon Resonance Sensing for Enhancing Protein Signals with a Simple Kretschmann Configuration. Sensors. 2022; 22(3):854. https://doi.org/10.3390/s22030854
Chicago/Turabian StyleTerao, Kyohei, and Shohei Kondo. 2022. "AC-Electroosmosis-Assisted Surface Plasmon Resonance Sensing for Enhancing Protein Signals with a Simple Kretschmann Configuration" Sensors 22, no. 3: 854. https://doi.org/10.3390/s22030854
APA StyleTerao, K., & Kondo, S. (2022). AC-Electroosmosis-Assisted Surface Plasmon Resonance Sensing for Enhancing Protein Signals with a Simple Kretschmann Configuration. Sensors, 22(3), 854. https://doi.org/10.3390/s22030854