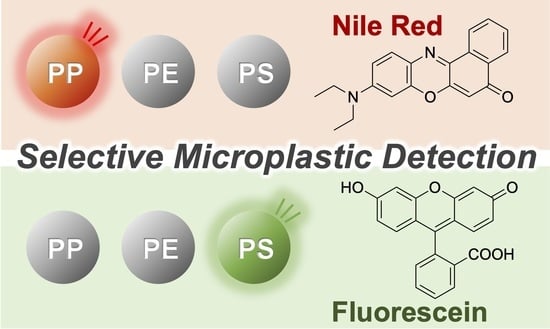
Material-Specific Determination Based on Microscopic Observation of Single Microplastic Particles Stained with Fluorescent Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Samples
2.3. Fluorescent Microscopic Imaging
2.4. Confocal Laser Microscopic Imaging
2.5. Analysis of Fluorescent Intensities of Stained Particles
3. Results
3.1. Fluorescent and Confocal Laser Microscopic Imaging
3.2. Intensity of Fluorescence of Microplastics Stained with Fluorescent Dyes
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Betts, K. Why small plastic particles may pose a big problem in the oceans. Environ. Sci. Technol. 2008, 42, 8995. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the Aquatic Environment. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: New York, NY, USA, 2015; pp. 325–340. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.; Yeo, M.-K. The environmental effects of microplastics on aquatic ecosystems. Mol. Cell. Toxicol. 2018, 14, 353–359. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2020, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Thomposon, R.C. Microplastics in the Ocean. Arch. Environ. Contam. Toxicol. 2015, 69, 215–268. [Google Scholar] [CrossRef] [PubMed]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Kross, S.M.; Armstrong, J.B.; Bogan, M.T.; Darling, E.S.; Green, S.J.; Smyth, A.R.; Veríssimo, D. Scientific Evidence Supports a Ban on Microbeads. Environ. Sci. Technol. 2015, 49, 10759–10761. [Google Scholar] [CrossRef] [Green Version]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Ranjan, V.P.; Joseph, A.; Goel, S. Microplastics and other harmful substances released from disposable paper cups into hot water. J. Hazard. Mater. 2021, 404, 124118. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested Microscopic Plastic Translocates to the Circulatory System of the Mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Setälä, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Phil. Trans. R. Soc. B 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhargava, R.; Wang, S.-Q.; Koenig, J.L. FTIR Microspectroscopy of Polymeric Systems. Adv. Polym. Sci. 2003, 163, 137–191. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.P.; Ojeda, J.J.; Romero-González, M.E. The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments. Sci. Total Environ. 2012, 416, 455–463. [Google Scholar] [CrossRef]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef] [Green Version]
- Käppler, A.; Windrich, F.; Löder, M.G.J.; Malanin, M.; Fischer, D.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Identification of microplastics by FTIR and Raman microscopy: A novel silicon filter substrate opens the important spectral range below 1300 cm−1 for FTIR transmission measurements. Anal. Bioanal. Chem. 2015, 407, 6791–6801. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Kuczera, M.; Mintenig, S.; Lorenz, C.; Gerdts, G. Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ. Chem. 2015, 12, 563–581. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but found with Nile Red: A novel method for detecting and quantifying small microplastics (1 mm to 20 μm) in environmental samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiggin, K.J.; Holland, E.B. Validation and application of cost and time effective methods for the T detection of 3–500 μm sized microplastics in the urban marine and estuarine environments surrounding Long Beach, California. Mar. Pollut. Bull. 2019, 143, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Reis, V.; Matos, J.T.V.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). Sci. Total Environ. 2019, 690, 1277–1283. [Google Scholar] [CrossRef]
- Tong, H.; Jiang, Q.; Zhong, X.; Hu, X. Rhodamine B dye staining for visualizing microplastics in laboratory-based studies. Environ. Sci. Pollut. Res. 2021, 28, 4209–4215. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Torimura, M.; Habe, H. Spectroscopic investigation of increased fluorescent intensity of fluorescent dyes when adsorbed onto polystyrene microparticles. Anal. Sci. 2021, 37, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Bujdák, J. The effects of layered nanoparticles and their properties on the molecular aggregation of organic dyes. J. Photochem. Photobiol. C 2018, 35, 108–133. [Google Scholar] [CrossRef]
- Giovannini, G.; Rossi, R.M.; Boesel, L.F. Changes in Optical Properties upon Dye–Clay Interaction: Experimental Evaluation and Applications. Nanomaterials 2021, 11, 197. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 2nd ed.; Academic Press: London, UK, 1994. [Google Scholar]
- Sangster, J. Octanol-Water Partition Coefficients of Organic Compounds. J. Phys. Chem. Ref. Data 1989, 18, 1111–1227. [Google Scholar] [CrossRef]
- PubChem. Nile Red. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nile-red (accessed on 28 February 2022).
- PubChem. Fluorescein. Available online: https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2128 (accessed on 28 February 2022).
- Lahnstein, K.; Schmehl, T.; Rüsch, U.; Rieger, M.; Seeger, W.; Gessler, T. Pulmonary absorption of aerosolized fluorescent markers in the isolated rabbit lung. Int. J. Pharm. 2008, 351, 158–164. [Google Scholar] [CrossRef]
- Magenau, A.J.D.; Richards, J.A.; Pasquinelli, M.A.; Savin, D.A.; Mathers, R.T. Systematic Insights from Medicinal Chemistry to Discern the Nature of Polymer Hydrophobicity. Macromolecules 2015, 48, 7230–7236. [Google Scholar] [CrossRef]






| (A) | ||||||
| PS | PE | PP | ||||
| Average | S. D. 1 | Average | S. D. 1 | Average | S. D. 1 | |
| Nile Red | 57.85 | 2.797 | 52.49 | 2.150 | 163.8 | 3.277 |
| Fluorescein | 80.02 | 1.542 | 52.49 | 3.155 | 66.43 | 2.162 |
| Rhodamine 6G | 60.86 | 2.401 | 59.14 | 2.829 | 51.87 | 0.9582 |
| Non-stained | 43.37 | 2.951 | 44.26 | 5.144 | 42.05 | 0.8222 |
| (B) | ||||||
| PS | PE | PP | ||||
| Average | S. D. 2 | Average | S. D. 2 | Average | S. D. 2 | |
| Nile Red | 14.48 | 4.066 | 8.234 | 5.575 | 121.7 | 3.379 |
| Fluorescein | 36.65 | 3.330 | 21.17 | 6.034 | 24.38 | 2.313 |
| Rhodamine 6G | 17.49 | 3.804 | 14.88 | 5.871 | 9.811 | 1.263 |
| Molecules | log Po/w | Reference |
|---|---|---|
| Nile Red | 3.8 | [31] |
| Fluorescein | 3.35 | [32] |
| Rhodamine 6G | 2.69 | [33] |
| PP 3 | 4.15 | [34] |
| PS 3 | 7.55 | [34] |
| PE 3 | 3.13 | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoki, H. Material-Specific Determination Based on Microscopic Observation of Single Microplastic Particles Stained with Fluorescent Dyes. Sensors 2022, 22, 3390. https://doi.org/10.3390/s22093390
Aoki H. Material-Specific Determination Based on Microscopic Observation of Single Microplastic Particles Stained with Fluorescent Dyes. Sensors. 2022; 22(9):3390. https://doi.org/10.3390/s22093390
Chicago/Turabian StyleAoki, Hiroshi. 2022. "Material-Specific Determination Based on Microscopic Observation of Single Microplastic Particles Stained with Fluorescent Dyes" Sensors 22, no. 9: 3390. https://doi.org/10.3390/s22093390
APA StyleAoki, H. (2022). Material-Specific Determination Based on Microscopic Observation of Single Microplastic Particles Stained with Fluorescent Dyes. Sensors, 22(9), 3390. https://doi.org/10.3390/s22093390