Room Temperature Ammonia Gas Sensing Using Mixed Conductor based TEMPOS Structures
Abstract
:1. Introduction
1.1. Ammonia sensors
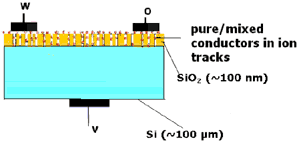
1.2. TEMPOS structures
1.3. Ion/Mixed conductor
1.4. Techniques used for ammonia sensing
2. Results and Discussion
3. Experimental Section
3.1. Sample preparation
3.2. The measuring setup
4. Conclusions
Acknowledgments
References and Notes
- St. Leger, R.J.; Nelson, J.O.; Screen, S.E. The entomopathogenic fungus Metarhizium anisopliae alters ambient pH, allowing extracellular protease production and activity. Microbiology 1999, 145, 2691–2699. [Google Scholar]
- Licyayo, D. C.M.; Suzuki, A.; Matsumoto, M. Interactions among ammonia fungi on MY agar medium with varying pH. Mycoscience 2007, 48, 20–28. [Google Scholar]
- Imamura, A.; Yumoto, T. Dynamics of fruit body production and mycorrhiza formation of ectomycorrhiza ammonia fungi in warm temperature forest in Japan. Mycoscience 2008, 49, 42–55. [Google Scholar]
- Ingeloeg, T.; Nohrstedt, H.-Oe. Ammonia formation and soil pH caused by decomposing fruit-bodies of macrofungi. Oecologia 1993, 93, 449–451. [Google Scholar]
- Kohl, D. Function and applications of gas sensors, topical review. J. Phys. D 2001, 34, R125–R149. [Google Scholar]
- Timmer, B.; Olthuis, W.; Berg, A.V.D. Ammonia sensors and their applications - a review. Sens. Actuat. B 2005, 107, 666–677. [Google Scholar]
- Chabukswar, V.V.; Pethkar, S.; Athawale, A.A. Acrylic acid doped polyaniline as an ammonia sensor. Sens. Actuat. B 2001, 77, 657–663. [Google Scholar]
- Raimundo, I.M.; Narayanaswamy, R. Simultaneous determination of relative humidity and ammonia in air employing an optical fibre sensor and artificial neural network. Sens. Actuat. B 2001, 74, 60–68. [Google Scholar]
- Lahdesaki, I.; Lewenstam, A.; Ivaska, A. A polypyrrole-based amperometric ammonia sensor. Talanta 1996, 43, 125–134. [Google Scholar]
- Zakrzewska, K. Mixed oxides as gas sensors. Thin Solid Films 2001, 391, 229–238. [Google Scholar]
- Wang, Y.D.; Wu, X.H.; Su, Q.; Li, Y.F.; Zhou, Z.L. Ammonia-sensing characteristics of Pt and SiO2 doped SnO2 materials. Solid-State Electron. 2001, 45, 347–350. [Google Scholar]
- Zhang, T.; Shen, Y.; Zhang, R.; Liu, X. Ammonia–sensing characteristics of Pt doped CdSnO3 semiconducting ceramic sensor. Mater. Lett. 1996, 27, 161–164. [Google Scholar]
- Shimizu, Y.; Okamato, T.; Takao, Y.; Egashira, M. Desorption behaviour of ammonia from TiO2 based specimens–ammonia sensing mechanism of double layer sensors with TiO2 based catalyst layers. J. Mol. Catal., A 2000, 155, 183–191. [Google Scholar]
- Guo, P.; Pan, H. Selectivity of Ti-doped In2O3 ceramics as an ammonia sensor. Sens. Actuators, B 2006, 114, 762–767. [Google Scholar]
- Clifford, P.K.; Tuma, D.T. Characteristics of semiconductor gas sensors I. Steady state gas response. Sens. Actuat. B 1983, 3, 233–254. [Google Scholar]
- Srivastava, R.K.; Lal, P.; Dwivedi, R.; Srivastava, S.K. Sensing mechanism in tin oxide–based thick film gas sensors. Sens. Actuat. B 1994, 21, 213–218. [Google Scholar]
- Xu, C.N.; Miura, N.; Ishida, Y.; Matuda, K.; Yamazoe, N. Selective detection of NH3 over NO in combustion exhausts by using Au and MoO3 doubly promoted WO3 element. Sens. Actuat. B 2000, 65, 163–164. [Google Scholar]
- Coutinho, C.F.B.; Muxel, A.A.; Rocha, C.G.; de Jesus, D.A.; Alfaya, R.V.S.; Almeida, F.A.S.; Y. Gushikem, Y.; Alfaya, A.A.S. Ammonium ion sensor based on SiO2/ZrO2/Phosphate-NH4+ composite for quantification of ammonium ions in natural waters. J. Braz. Chem. Soc. 2007, 18, 189–194. [Google Scholar]
- Delvaux, M.; Stavaux, P.-Y.; Dauginet-de-Pra, L.; Dehaye, F.; Cuenot, S.; Demoustier- Champagne, S.; Nysten, B.; Ferain, E.; Legras, R. Chemical modifications induced in bisphenol A polycarbonate by swift heavy ions. 5th Int. Symp. SHIM, Giordano Naxos, Italy, May 22– 25, 2002.
- Srivastava, A.; Singh, V.; Dhand, C.; Kaur, M.; Singh, T.; Witte, K.; Scherer, U.W. Study of Swift heavy ion modified conducting polymer composites for application as gas sensors. Sensors 2006, 6, 262–269. [Google Scholar]
- Fink, D.; Chadderton, L.T.; Kiv, A.; Saad, A.; Tabacnics, M.; Rizutto, M. de A.; Silva, A.DE O.D.; Fahrner, W. R.; Hoppe, K. Radiation Eff. Def. Solids.; 2007; Volume 162, pp. 543–555. [Google Scholar]
- Fink, D.; Petrov, A.V.; Hoppe, K.; Fahrner, W.R.; Papaleo, R.M.; Berdinsky, A.S.; Chandra, A.; Chemseddine, A.; Zrineh, A.; Biswas, A.; Faupel, F.; Chadderton, L.T. Etched ion tracks in silicon oxide and silicon oxynitride as charge injection or extraction channels for novel electronic structures. Nucl. Instr. Meth. Phys. Res. B 2004, 218, 355–361. [Google Scholar]
- Sinha, D.; Petrov, A.; Fink, D.; Fahrner, W.R.; Hoppe, K.; Chandra, A. TEMPOS Structures with Gold Nanoclusters. Radiation Eff. Def. Solids. 2004, 159, 517–533. [Google Scholar]
- Fink, D.; Petrov, A.V.; Rao, V.; Wilhelm, M.; Demyanov, S.; Szimkoviak, P.; Behar, M.; Alegaonkar, P.S.; Chadderton, L.T. Production parameters for the formation of metallic nanotubules in etched tracks. Radia. Meas. 2003, 36, 751–755. [Google Scholar]
- Fink, D.; Alegaonkar, P.S.; Petrov, A.V.; Wilhelm, M.; Szimkowiak, P.; Behar, M.; Sinha, D.; Fahrner, W.R.; Hoppe, K.; Chadderton, L. High energy ion beam irradiation of polymers for electronic applications. Nucl. Instr. Meth. Phys. Res. B. 2005, 236, 11–20. [Google Scholar]
- Fink, D.; Chandra, A.; Fahrner, W.R.; Hoppe, K.; Opitz-Coutureau, J; Bundesmann, J. Resistance of ion track based electronic structures to high voltage and radiation. J. Phys. D: Appl. Phys. 2007, 40, 3212–3218. [Google Scholar]
- Fink, D.; Chandra, A.; Opitz-Coutureau, J; Fahrner, W.R.; Hoppe, K.; Papaleo, R.M. Tunable electronically anisotropic materials with ion-irradiated polysilanes on semiconductor. Appl. Phys. A 2007, 86, 469–476. [Google Scholar]
- Hashmi, S.A.; Kumar, A.; Maurya, K.K.; Chandra, S. Proton–conducting polymer electrolyte I: The polyethylene oxide + NH4ClO4 system. J. Phys. D 1990, 23, 1307–1314. [Google Scholar]
- Singh, P.K.; Chandra, S.; Chandra, A. Polymer electrolyte composites with dispersed semiconductors. J. Mat. Sci. Lett. 2002, 21, 1393–1395. [Google Scholar]
- Kumar, M.; Chandra, A. Studies on semiconductor dispersed polymer electrolyte composite (PEO: NH4ClO4+Bi2S3). Phys. Stat. Sol. A 2008, 205, 188–193. [Google Scholar]
- Chandra, A.; Singh, P.K.; Chandra, S. Semiconductor-dispersed polymer electrolyte composites. Solid State Ionics 2002, 154-155, 15–20. [Google Scholar]
- Fink, D.; Chadderton, L.T. Ion-solid interactions: current status, new perspectives. Radiat. Eff. Def. Solids 2005, 160, 67–83. [Google Scholar]
- Varghese, O.K.; Gong, D.; Paulose, M.; Ong, K.G.; Grimes, C.A.; Dickey, E.C. Highly ordered nanoporous alumina films: Effect of pore size and uniformity on sensing performance. J. Mater. Res. 2002, 17, 1162–1171. [Google Scholar]
- Dickey, E.C.; Varghese, O.K.; Ong, K.G.; Gong, D.; Paulose, M.; Grimes, C. A. Room temperature ammonia and humidity sensing using highly ordered nanoporous alumina films. Sensors 2002, 2, 91–110. [Google Scholar]
- Varghese, O. K.; Grimes, C. A. Metal Oxide Nanoarchitectures for Environmental Sensing. J. Nanosci. Nanotech. 2005, 3, 277–293. [Google Scholar]
- Varghese, O.K.; Gong, D.; Dreschel, W.R.; Ong, K.G.; Grimes, C.A. Ammonia detection using nanoporous alumina resistive and surface acoustic wave sensors. Sens. Actuat. B 2003, 94, 27–35. [Google Scholar]
- Varghese, O.K.; Kichambre, P.D.; Gong, D.; Ong, K.G.; Dickey, E.C.; Grimes, C.A. Gas sensing characteristics of multi-wall carbon nanotubes. Sens. Actuat. B 2001, 81, 32–41. [Google Scholar]
- Saroch, M.; Srivastava, S.; Fink, D.; Chandra, A. TEMPOS devices as humidity sensors. Radiat. Eff. Def. Solids 2008, 163, 645–653. [Google Scholar]
- Martin, C.R. Nanomaterials: A Membrane-Based Synthetic Approach. Science 1994, 266, 1961–1966. [Google Scholar]












| NH3phase to be detected | Detector type | Working medium | References |
|---|---|---|---|
| Gaseous | Insulating polymer | Bisphenol-A-polycarbonate | [19] |
| Conducting polymer | Polyamide | [5, 7] | |
| Nafion | [8] | ||
| Polypyrrole | as-received [9] SHI modified [19] | ||
| Metal oxide | SnO2 | [10, 11 ] | |
| CdSnO3 | [12] | ||
| TiO2 | [13] | ||
| In2O3 | [14] | ||
| Catalytic (based on change in charge carrier conc. in the metal) | Pd | [6] | |
| TEMPOS | PEO:NH4ClO4-filled etched tracks in SiO2 or SiON on Si | This work | |
| Liquid | Optical (Spectrophotometric NH3 detection based on pH change) | Nessler reagent (dipotassium tetraiodomercurate in NaOH) Berthelot reagent (phenol + hypochlorite) | [6] |
| Sensor type | (Ir)reversibility | Detection threshold | Response time[s] | Remarks | Working temperature (°C) and structure |
|---|---|---|---|---|---|
| Conducting polymer (PPy) | Irreversible | 10-6 | ∼ 250 | Sensitivity decreases with time | Up to 150°C |
| Metal oxides (WO3) | Reversible | 10-6 | ∼300 | Low selectivity drift | 400°C, rugged, inexpensive |
| Catalytic (Palladium) | Reversible | 10-6 | ∼ 60 | Limited accuracy, selectivity depends on temperature | Up to 600°C |
| Optical: (Nessler) | Irreversible | 10-9 | 60 | All are sensitive and selective and have expensive setup | 37°C |
| (Coulorometric) | 10-12 | 300 | |||
| (Absorption spectroscopy) | 10-9 | 300 | |||
| SHI-modified IPCP | Reversible | 10-2 | ∼500 | Sensitivity ion-fluence dependent | Room temp. (25-28°C), inexpensive set up |
| This work: TEMPOS sensor | Reversible | ∼10-5 | ∼0.2 | Sensitivity depends on frequency, material in tracks and voltage of applied signal | Room temperature (28°C), inexpensive set up |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saroch, M.; Srivastava, S.; Fink, D.; Chandra, A. Room Temperature Ammonia Gas Sensing Using Mixed Conductor based TEMPOS Structures. Sensors 2008, 8, 6355-6370. https://doi.org/10.3390/s8106355
Saroch M, Srivastava S, Fink D, Chandra A. Room Temperature Ammonia Gas Sensing Using Mixed Conductor based TEMPOS Structures. Sensors. 2008; 8(10):6355-6370. https://doi.org/10.3390/s8106355
Chicago/Turabian StyleSaroch, Mamta, Sunita Srivastava, Dietmar Fink, and Amita Chandra. 2008. "Room Temperature Ammonia Gas Sensing Using Mixed Conductor based TEMPOS Structures" Sensors 8, no. 10: 6355-6370. https://doi.org/10.3390/s8106355
APA StyleSaroch, M., Srivastava, S., Fink, D., & Chandra, A. (2008). Room Temperature Ammonia Gas Sensing Using Mixed Conductor based TEMPOS Structures. Sensors, 8(10), 6355-6370. https://doi.org/10.3390/s8106355