Effects of Gold Nanoparticles on the Response of Phenol Biosensor Containing Photocurable Membrane with Tyrosinase
Abstract
:1. Introduction
2. Methodology
2.1 Reagents
2.2 Apparatus and measurements
2.3 Preparation of phenol biosensor
2.4 Electrochemical measurements
3. Results and Discussion
3.1 The linear response slope of the phenol biosensor
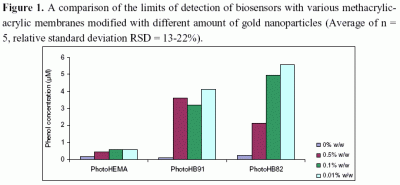
3.2 Effets of gold nano-particles on the detection limit of the phenol biosensors
3.3 Effects of gold nanoparticles on the linear response range for phenol detection
3.4. Effects of gold nanoparticles on the response time of the biosensors to phenol
4. Conclusion
Acknowledgments
References
- Liu, S.; Yu, J.; Ju, H. Renewable phenol biosensor based on tyrosinase-colloidal gold modified carbon paste electrode. J. Electroanal. Chem. 2003, 540, 61–67. [Google Scholar]
- Zhou, X.; Yu, T.; Zhang, Y.; Kong, J.; Tang, Y.; Marty, J.L.; Liu, B. Nanozeolite-assembled interface towards sensitive biosensing. Electrochem. Commun. 2007, 9, 525–1529. [Google Scholar]
- Kong, Y.T.; Boopathi, M.; Shim, Y.B. Direct electrochemistry of horseradish peroxidase bonded on a conducting polymer modified glassy carbon electrode. Biosens. Bioelectron. 2003, 19, 227–232. [Google Scholar]
- Sanz, V.C.; Mena, M.L.; Cortés, A.G.; Sedeńo, P.Y.; Pingarrón, J.M. Development of a tyrosinase biosensor based on gold nanoparticles-modified glassy carbon electrodes. Application to the measurement of a bioelectrochemical polyphenols index in wines. Anal. Chim. Acta. 2005, 528, 1–8. [Google Scholar]
- Chen, J.; Tang, J.; Ju, H. A gold nanoparticles/sol–gel composite architecture for encapsulation of immunoconjugate for reagentless electrochemical immunoassay. Biomaterials 2006, 27, 2313–2321. [Google Scholar]
- Njagi, J.; Andreescu, S. Stable enzyme biosensors based on chemically synthesized Au– polypyrrole nanocomposites. Biosens. Bioelectron. 2007, 23, 168–175. [Google Scholar]
- Mengmeng, G.; Yunhui, Y.; Zhijie, W.; Guoli, S.; Ruqin, Y. A mediator-free horseradish peroxidase biosensor based on concanavalin A. Chinese J. Anal. Chem. 2006, 34, 399–402. [Google Scholar]
- Zhao, S.; Zhang, K.; Bai, Y.; Yang, W.; Sun, C. Glucose oxidase/colloidal gold nanoparticles immobilized in Nafion film on glassy carbon electrode: Direct electron transfer and electrocatalysis. Bioelectrochemistry 2006, 69, 158–163. [Google Scholar]
- Yang, M.H.; Qu, F.L.; Lu, Y.S.; Shen, G.L.; Yu, R.Q. In situ chemical reductive growth of platinum nanoparticles on glass slide for the mass fabrication of biosensors. Talanta 2008, 74, 831–835. [Google Scholar]
- Skoog, D.A.; Holler, F.J.; Nieman, T.A. Principles of Instrumental Analysis, 5th ed.; Thomson Learning Inc.: Philadelphia, USA, 1998. [Google Scholar]
- Bean, L.S.; Heng, L.Y.; Yamin, B.M.; Ahmad, M. Photocurable ferrocene-containing poly(2-hydroxyl ethyl methacrylate) films for mediated amperometric glucose biosensor. Thin Solid Films 2005, 477, 104–110. [Google Scholar]
- Bean, L. S.; Heng, L. Y.; Yamin, B. M.; Ahmad, M. The electrochemical behaviour of ferrocene in a photocurable poly(methyl methacrylate-co-2-hydroxylethyl methacrylate) film for a glucose biosensor. Bioelectrochemistry 2005, 65, 157–165. [Google Scholar]
- Wang, B.; Dong, S. Organic-phase enzyme electrode for phenolic determination based on a functionalized sol-gel composite. J. Electroanal. Chem. 2000, 487, 45–50. [Google Scholar]
- Shan, D.; Zhu, M.; Han, E.; Xue, H.; Cosnier, S. Calcium carbonate nanoparticles: A host matrix for the construction of highly sensitive amperometric phenol biosensor. Biosens. Bioelectron. 2007, 23, 648–654. [Google Scholar]
- Fan, Q.; Shan, D.; Xue, H.; He, Y.; Cosnier, S. Amperometric phenol biosensor based on laponite clay-chitosan nanocomposite matrix. Biosens. Bioelectron. 2007, 22, 816–821. [Google Scholar]
- Jung, H.Y.; Park, Y.K.; Park, S.; Kim, S.K. Surface enhanced Raman scattering from layered assemblies of close-packed gold nanoparticles. Anal. Chimi. Acta. 2007, 602, 236–243. [Google Scholar]
- Barhoumi, H.; Maaref, A.; Rammah, M.; Marteleb, C.; Renault, N.J.; Moustyc, C.; Cosnier, S.; Perez, E.; Lattes, I.R. Short communication: Insulator semiconductor structures coated with biodegradable latexes as encapsulation matrix for urease. Biosens. Bioelectron. 2005, 20, 2318–2323. [Google Scholar]



| PhotoHEMA | PhotoHB91 | PhotoHB82 | ||||
|---|---|---|---|---|---|---|
| Gold nano- particles(% w/w) | Slope (μA/μM) | R2 (n=17) | Slope (μA/μM) | R2 (n=10-14) | Slope (μA/μM) | R2 (n=7-12) |
| 0 | 0.03 | 0.9769 | 0.030 | 0.9946 | 0.02 | 0.9789 |
| 0.01 | 0.02 | 0.9886 | 0.005 | 0.9924 | 0.0007 | 0.9877 |
| 0.1 | 0.03 | 0.9912 | 0.007 | 0.9847 | 0.0006 | 0.9862 |
| 0.5 | 0.03 | 0.9937 | 0.008 | 0.9893 | 0.0007 | 0.9900 |
| Linear concentration range of phenol (μM) | |||
|---|---|---|---|
| Gold nanoparticles (% w/w) | PhotoHEMA (n=17) | PhotoHB91 (n=10-14) | PhotoHB82 (n=7-12) |
| 0 | 6.2 – 42.2 | 6.2 – 48.2 | 6.2 – 24.2 |
| 0.01 | 6.2 – 90.2 | 6.2 – 60.2 | 6.2 – 42.2 |
| 0.1 | 6.2 – 90.2 | 6.2 – 66.2 | 6.2 – 60.2 |
| 0.5 | 6.2 – 90.2 | 6.2 – 90.2 | 6.2 – 72.2 |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sharina, A.H.; Lee, Y.H.; Musa, A. Effects of Gold Nanoparticles on the Response of Phenol Biosensor Containing Photocurable Membrane with Tyrosinase. Sensors 2008, 8, 6407-6416. https://doi.org/10.3390/s8106407
Sharina AH, Lee YH, Musa A. Effects of Gold Nanoparticles on the Response of Phenol Biosensor Containing Photocurable Membrane with Tyrosinase. Sensors. 2008; 8(10):6407-6416. https://doi.org/10.3390/s8106407
Chicago/Turabian StyleSharina, Abu Hanifah, Yook Heng Lee, and Ahmad Musa. 2008. "Effects of Gold Nanoparticles on the Response of Phenol Biosensor Containing Photocurable Membrane with Tyrosinase" Sensors 8, no. 10: 6407-6416. https://doi.org/10.3390/s8106407
APA StyleSharina, A. H., Lee, Y. H., & Musa, A. (2008). Effects of Gold Nanoparticles on the Response of Phenol Biosensor Containing Photocurable Membrane with Tyrosinase. Sensors, 8(10), 6407-6416. https://doi.org/10.3390/s8106407