NeuroMEMS: Neural Probe Microtechnologies
Abstract
:1. Introduction
2. Background for Neural Probes
3. Metal Wire Based Neural Probes
4. Silicon Based Neural Probes
4.1. Silicon on Insulator (SOI) based Neural Probes
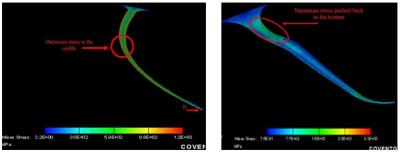
4.2. Standard Commercial MEMS Process based probes
5. Polymer based neural probes
6. Biocompatibility of Neural Probes
7. Conclusions
Acknowledgments
References and Notes
- Franklin, B. An account of the effects of electricity in paralytic cases. Philos. Trans. (1683-1775) 1757, 50, 481–483. [Google Scholar]
- McNeal, D.R. 2000 years of electrical stimulation. Functional Electrical Stimulation Applications in Neural Prostheses Biomedical Engineering and Instrumentation Series 1977, 3, 3–35. [Google Scholar]
- Hodgkin, A.L.; Huxley, A.F. Action potentials recorded from inside a nerve fibre. Nature 1939, 144, 710–711. [Google Scholar]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol.-London 1952, 117, 500–544. [Google Scholar]
- Huxley, A.F. Hodgkin and the action potential 1935-1952. J. Physiol.-London 2002, 538, 2–2. [Google Scholar]
- Gesteland, R.C.; Howland, B.; Lettvin, J.Y.; Pitts, W.H. Comments on microelectrodes. Proc. Inst. Radio Eng. 1959, 47, 1856–1862. [Google Scholar]
- Frank, K.; Becker, M.C. Microelectrodes for recording and stimulation. In Physical Techniques in Biological Research.; Nastuk, W.L., Ed.; Academic Press: New York, 1964; Volume 5, pp. 23–88. [Google Scholar]
- Geddes, L.A. Electrodes and the measurement of bioelectric events; Wiley-Interscience: New York, 1972. [Google Scholar]
- Nicolelis, M.A.L. Brain-machine interfaces to restore motor function and probe neural circuits. Nature Rev. Neurosci. 2003, 4, 417–422. [Google Scholar]
- Rutten, W.L.C. Selective electrical interfaces with the nervous system. Annu. Rev. Biomed. Eng. 2002, 4, 407–452. [Google Scholar]
- Najafi, K. Solid-state microsensors for cortical nerve recordings. IEEE Eng. Med. Biol. Mag. 1994, 13, 375–87. [Google Scholar]
- Najafi, K. Micromachined systems for neurophysiological applications. In Handbook of microlithography, micromachining, and microfabrication.; SPIE-International Society for Optical Engine: Chicago, 1997; pp. 517–569. [Google Scholar]
- Heiduschka, P.; Thanos, S. Implantable bioelectronic interfaces for lost nerve functions. Prog. Neurobiol. 1998, 55, 433–461. [Google Scholar]
- Stieglitz, T.; Meyer, J.U. Microtechnical interfaces to neurons. Microsys. Tech. Chem. Life Sci. 1998, 194, 131–162. [Google Scholar]
- Hubel, D.H.; Wiesel, T.N. (Eds.) Ferrier Lecture: Functional architecture of macaque monkey visual cortex. In Proc. R. Soc. Lond. B.; 1977; Volume 198, pp. 1–59.
- McLaughlin, D.; Shapley, R.; Shelley, M.; Wielaard, D.J. A neuronal network model of macaque primary visual cortex (V1): Orientation selectivity and dynamics in the input layer 4C alpha. PNAS 2000, 97, 8087–8092. [Google Scholar]
- Gilbert, C.D. Adult cortical dynamics. Physiol. Rev. 1998, 78, 467–485. [Google Scholar]
- Deadwyler, S.A.; Hampson, R.E. The significance of neural ensemble codes during behavior and cognition. Annu. Rev. Neurosci. 1997, 20, 217–244. [Google Scholar]
- Nicolelis, M.A.L. Methods for Neural Ensemble Recordings, 1st Edition ed; CRC-Press: New York, 1998. [Google Scholar]
- Normann, R.A.; Warren, D.J.; Ammermuller, J.; Fernandez, E.; Guillory, S. High-resolution spatio-temporal mapping of visual pathways using multi-electrode arrays. Vision Res. 2001, 41, 1261–75. [Google Scholar]
- Nicolelis, M.A.L.; Ribeiro, S. Multiellectrode recordings: the next steps. Curr. Opin. Neurobiol. 2002, 12, 602–606. [Google Scholar]
- Hoffman, K.L.; McNaughton, B.L. Coordinated reactivation of distributed memory traces in primate neocortex. Science 2002, 297, 2070–2073. [Google Scholar]
- Nirenberg, S.; Latham, P.E. Population coding in the retina. Curr. Opin. Neurobiol. 1998, 8, 488–493. [Google Scholar]
- Vos, B.P.; Wijnants, M.; Taeymans, S.; De Schutter, E. Miniature carrier with six independently moveable electrodes for recording of multiple single-units in the cerebellar cortex of awake rats. J. Neurosci. Meth. 1999, 94, 19–26. [Google Scholar]
- Grumet, A.E.; Wyatt, J.L.; Rizzo, J.F. Multi-electrode stimulation and recording in the isolated retina. J. Neurosci. Meth. 2000, 101, 31–42. [Google Scholar]
- Wilson, M.A.; McNaughton, B.L. Dynamics of the hippocampal ensemble code for space. Science 1993, 261, 1055–1058. [Google Scholar]
- Petersson, P.; Holmer, M.; Breslin, T.; Granmo, M.; Schouenborg, J. An imaging system for monitoring receptive field dynamics. J. Neurosci. Meth. 2001, 104, 123–131. [Google Scholar]
- Rousche, P.J.; Petersen, R.S.; Battiston, S.; Giannotta, S.; Diamond, M.E. Examination of the spatial and temporal distribution of sensory cortical activity using a 100-electrode array. J. Neurosci. Meth. 1999, 90, 57–66. [Google Scholar]
- Petersen, R.S.; Diamond, M.E. Spatial-temporal distribution of whisker-evoked activity in rat somatosensory cortex and the coding of stimulus location. J. Neurosci. 2000, 20, 6135–6143. [Google Scholar]
- Ghazanfar, A.A.; Stambaugh, C.R.; Nicolelis, M.A.L. Encoding of tactile stimulus location by somatosensory thalamocortical ensembles. J. Neurosci. 2000, 20, 3761–2000. [Google Scholar]
- Neuenschwander, S.; Castelo-Branco, M.; Singer, W. Synchronous oscillations in the cat retina. Vision Res. 1999, 39, 2485–97. [Google Scholar]
- Prechtl, J.C. Visual-motion induces synchronous oscillations in turtle visual-cortex. PNAS 1994, 91, 12467–12471. [Google Scholar]
- Prechtl, J.C.; Cohen, L.B.; Pesaran, B.; Mitra, P.P.; Kleinfeld, D. Visual stimuli induce waves of electrical activity in turtle cortex. PNAS 1997, 94, 7621–7626. [Google Scholar]
- Gagne, S.; Plamondon, R. Open tip glass microelectrodes - conduction through the wall at the tip. IEEE Trans. Biomed. Eng. 1987, 34, 56–61. [Google Scholar]
- Schanne, O.F.; Lavallee, M.; Laprade, R.; Gagne, S. Electrical properties of glass microelectrodes. Proc. IEEE 1968, 56, 1072–1082. [Google Scholar]
- Chowdhury, Tk. Fabrication of extremely fine glass micropipette electrodes. J. Phys. E-Sci. Instrum. 1969, 2, 1087–1090. [Google Scholar]
- Kennard, D.W. Glass microcapillary electrodes used for measuring potential in living tissues. In Electronic Apparatus for Biological Research.; Butterworths Scientific Publications: London, 1958; pp. 534–567. [Google Scholar]
- Robinson, D.A. The electrical properties of metal microelectrodes. Proc. IEEE 1968, 56, 1065–1071. [Google Scholar]
- Skrzypek, J.; Keller, E. Manufacture of metal microelectrodes with scanning electron-microscope. IEEE Trans. Biomed. Eng. 1975, 22, 435–437. [Google Scholar]
- Terzuolo, C.A.; Araki, T. An analysis of intra-versus extracellular potential changes associated with activity of single spinal motoneurons. Ann. NY. Acad. Sci. 1961, 94, 547–558. [Google Scholar]
- Scherberger, H.; Fineman, I.; Musallam, S.; Dubowitz, D.J.; Bernheim, K.A.; Pesaran, B. Magnetic resonance image-guided implantation of chronic recording electrodes in the macaque intraparietal sulcus. J. Neurosci. Meth. 2003, 130, 1–8. [Google Scholar]
- Tsytsarev, V.; Taketani, M.; Schottler, F.; Tanaka, S.; Hara, M. A new planar multielectrode array: recording from a rat auditory cortex. J. Neural Eng. 2006, 3, 293–298. [Google Scholar]
- Musallam, S.; Bak, M.J.; Troyk, P.R.; Andersen, R.A. A floating metal microelectrode array for chronic implantation. J. Neurosci. Meth. 2007, 160, 122–127. [Google Scholar]
- Wise, K.D.; Angell, J.B.; Starr, A. An integrated-circuit approach to extracellular microelectrodes. IEEE Trans. Biomed. Eng. 1970, BME-17, 238–247. [Google Scholar]
- Wise, K.D.; Najafi, K. Microfabrication Techniques for Integrated Sensors and Microsystems. Science 1991, 254, 1335–1342. [Google Scholar]
- Banks, D.; Ewins, D.J.; Balachandran, W.; Richards, P.R. Microengineered interfaces with the nervous system. IEEE Colloqu. Med. Appl. Microeng. 1996, 4, 1–4. [Google Scholar]
- Urban, G.A.; Prohaska, O.; Olcaytug, F. BioMEMS; Springer: Chicago, US, 2006; Chapter 1(Early BioMEMS Multi-Sensor Neuroprobes); pp. 1–13. [Google Scholar]
- Banks, D. Neurotechnology. Eng. Sci. 1998, 7, 135–144. [Google Scholar]
- Pearce, T.M.; Williams, J.C. Microtechnology: meet neurobiology. Lab Chip 2007, 7, 30–40. [Google Scholar]
- Banks, D.; Ewins, D. Microengineered neural signal transducers for peripheral nerve. Current work and future requirements. Proc. 3rd Int. Conf. on Medical and biological implant technology, Nottingham, UK; 1996. [Google Scholar]
- Rousche, P.J.; Pellinen, D.S.; Pivin, D.P.; Williams, J.C.; Vetter, R.J.; Kipke, D.R. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans. Biomed. Eng. 2001, 48, 361–371. [Google Scholar]
- Metz, S.; Bertsch, A.; Bertrand, D.; Renaud, P. Flexible polyimide probes with microelectrodes and embedded microfluidic channels for simultaneous drug delivery and multi-channel monitoring of bioelectric activity. Biosens. Bioelectron. 2004, 19, 1309–1318. [Google Scholar]
- Stieglitz, T. Flexible biomedical microdevices with double-sided electrode arrangements for neural applications. Sens. Actuat. A-Phys. 2001, 90, 203–211. [Google Scholar]
- Stieglitz, T.; Gross, M. Flexible BIOMEMS with electrode arrangements on front and back side as key component in neural prostheses and biohybrid systems. Sens. Actuat. B-Chem. 2002, 83, 8–14. [Google Scholar]
- Stieglitz, T.; Beutel, H.; Keller, R.; Schuettler, M.; Meyer, J.-U. Flexible, polyimide-based neural interfaces. In Microelectronics for Neural, Fuzzy and Bio-Inspired Systems; Granada, Spain, 1999; pp. 112–119. [Google Scholar]
- Stieglitz, T.; Beutel, H.; Keller, R.; Schuettler, M.; Meyer, J.-U. Micromachined, polyimide-based devices for flexible neural interfaces. Biomed. Microdev. 2000, 2, 283–94. [Google Scholar]
- Navarro, X.; Calvet, S.; Rodriguez, F.J.; Stieglitz, T.; Blau, C.; Buti, M. Stimulation and recording from regenerated peripheral nerves through polyimide sieve electrodes. J. Peripher. Nerv. Syst. 1998, 3, 91–101. [Google Scholar]
- Suh, M.; Ma, H.T.; Zhao, M.R.; Sharif, S.; Schwartz, T.H. Neurovascular coupling and oximetry during epileptic events. Mol. Neurobiol. 2006, 33, 181–197. [Google Scholar]
- Donoghue, J.P. Connecting cortex to machines: recent advances in brain interfaces. Nat. Neurosci. 2002, 1085–1088. [Google Scholar]
- Wise, K.D. Silicon microsystems for neuroscience and neural prostheses. IEEE Eng. Med. Biol. Mag. 2005, 24, 22–29. [Google Scholar]
- Bear, M.R.; Connors, B. W.; Paradiso, M. A. Neuroscience Exploring the Brain., 2nd ed; Lippincott Williams and Wilkins: Baltimore, 2001. [Google Scholar]
- Takahashi, H.; Suzurikawa, J.; Nakao, M.; Mase, F.; Kaga, K. Easy-to-prepare assembly array of tungsten microelectrodes. IEEE Trans. Biomed. Eng. 2005, 52, 952–956. [Google Scholar]
- Schmidt, E.M.; Bak, M.J.; Christensen, P. Laser exposure of Parylene-C insulated microelectrodes. J. Neurosci. Meth. 1995, 62, 89–92. [Google Scholar]
- Xu, C.Y.; Lemon, W.; Liu, C. Design and fabrication of a high-density metal microelectrode array for neural recording. Sens. Actuat. A-Phys. 2002, 96, 78–85. [Google Scholar]
- Frazier, A.B.; O'Brien, D.P.; Allen, M.G. Two dimensional metallic microelectrode arrays for extracellular stimulation and recording of neurons. Proc. IEEE Micro. Electro. Mech. Syst. 1993, 195–200. [Google Scholar]
- Oka, H.; Shimono, K.; Ogawa, R.; Sugihara, H.; Taketani, M. A new planar multielectrode array for extracellular recording: application to hippocampal acute slice. J. Neurosci. Meth. 1999, 93, 61–67. [Google Scholar]
- Anon. Electrodeposition method creates 3-D microstructures. Chem. Eng. Prog. 2003, 99, 20. [Google Scholar]
- Motta, P.S.; Judy, J.W. Multielectrode microprobes for deep-brain stimulation fabricated with a customizable 3-D electroplating process. IEEE Trans. Biomed. Eng. 2005, 52, 923–933. [Google Scholar]
- Maciossek, A. Electrodeposition of 3-D microstructures without moulds. Proc SPIE. 1996, 2879, 275–279. [Google Scholar]
- Najafi, K. Micromachined systems for neurophysiological applications. In Handbook of Microlithography, Micromachining and Microfabrication, Volume II: Micromachining and Microfabrication; Rai-Choudhury, Prosenjit, Ed.; SPIE PRESS Monograph: London, 1997. [Google Scholar]
- Najafi, K.; Hetke, J.F. Strength characterization of silicon microprobes in neurophysiological tissues. IEEE Trans. Biomed. Eng. 1990, 37, 474–481. [Google Scholar]
- Muthuswamy, J.; Okandan, M.; Jain, T.; Gilletti, A. Electrostatic microactuators for precise positioning of neural microelectrodes. IEEE Trans. Biomed. Eng. 2005, 52, 1748–1755. [Google Scholar]
- Ji, J.; Najafi, K.; Wise, K.D. A scaled electronically-configurable multichannel recording array. Sens. Actuat. A-Phys. 1990, 22, 589–591. [Google Scholar]
- Tanghe, S.J.; Wise, K.D. A 16-channel CMOS neural stimulating array. IEEE J. Solid-State Circuits 1992, 27, 1819–1825. [Google Scholar]
- Ji, J.; Najafi, K.; Wise, K.D. A low-noise demultiplexing system for active multichannel microelectrode arrays. IEEE Trans. Biomed. Eng. 1991, 38, 75–81. [Google Scholar]
- Ji, J.; Wise, K.D. An implantable CMOS circuit interface for multiplexed microelectrode recording arrays. IEEE J. Solid-State Circuits 1992, 27, 433–443. [Google Scholar]
- Kim, C.; Wise, K.D. A 64-site multishank CMOS low-profile neural stimulating probe. IEEE J. Solid-State Circuits 1996, 31, 1230–1238. [Google Scholar]
- Kim, C.H.; Wise, K.D. Low-voltage electronics for the stimulation of biological neural networks using fully complementary BiCMOS circuits. IEEE J. Solid-State Circuits. 1997, 32, 1483–1490. [Google Scholar]
- Olsson, R.H.; Buhl, D.L.; Sirota, A.M.; Buzsaki, G.; Wise, K.D. Band-tunable and multiplexed integrated circuits for simultaneous recording and stimulation with microelectrode arrays. IEEE Trans. Biomed. Eng. 2005, 52, 1303–1311. [Google Scholar]
- Bai, Q.; Wise, K.D.; Anderson, D.J. A high-yield microassembly structure for three-dimensional microelectrode arrays. IEEE Trans. Biomed. Eng. 2000, 47, 281–289. [Google Scholar]
- Bai, Q.; Wise, K.D. Single-unit neural recording with active microelectrode arrays. IEEE Trans. Biomed. Eng. 2001, 48, 911–920. [Google Scholar]
- Yao, Y.; Gulari, M.N.; Ghimire, S.; Hetke, J. F.; Wise, K. D. A low-profile three-dimensional silicon/parylene stimulating electrode array for neural prosthesis applications. IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China; 2005. [Google Scholar]
- Cheung, K.C.; Djupsund, K.; Dan, Y.; Lee, L.P. Implantable multichannel electrode array based on SOI technology. J. Microelectromech. Syst. 2003, 12, 179–184. [Google Scholar]
- Wise, K.D.; Anderson, D.J.; Hetke, J.F.; Kipke, D.R.; Najafi, K. Wireless implantable microsystems: High-density electronic interfaces to the nervous system. Proc. IEEE 2004, 92, 76–97. [Google Scholar]
- Tanghe, S.J.; Najafi, K.; Wise, K.D. A Planar Iro Multichannel Stimulating Electrode for Use in Neural Prostheses. Sens. Actuat. B-Chem. 1990, 1, 464–467. [Google Scholar]
- Najafi, K.; Ji, J.; Wise, K.D. Scaling Limitations of Silicon Multichannel Recording Probes. IEEE Trans. Biomed. Eng. 1990, 37, 1–11. [Google Scholar]
- Drake, K.L.; Wise, K.D.; Farraye, J.; Anderson, D.J.; Bement, S.L. Performance of Planar Multisite Microprobes in Recording Extracellular Single-Unit Intracortical Activity. IEEE Trans. Biomed. Eng. 1988, 35, 719–732. [Google Scholar]
- Hoogerwerf, A.C.; Wise, K.D. A 3-Dimensional microelectrode array for chronic neural Recording. IEEE Trans. Biomed. Eng. 1994, 41, 1136–1146. [Google Scholar]
- Anderson, D.J.; Najafi, K.; Tanghe, S.J.; Evans, D.A.; Levy, K.L.; Hetke, J.F. Batch-fabricated thin-film electrodes for stimulation of the central auditory-system. IEEE Trans. Biomed. Eng. 1989, 36, 693–704. [Google Scholar]
- Chen, J.K.; Wise, K.D. A silicon probe with integrated microheaters for thermal marking and monitoring of neural tissue. IEEE Trans. Biomed. Eng. 1997, 44, 770–774. [Google Scholar]
- Hetke, J.F.; Lund, J.L.; Najafi, K.; Wise, K.D.; Anderson, D.J. Silicon ribbon cables for chronically implantable microelectrode arrays. IEEE Trans. Biomed. Eng. 1994, 41, 314–321. [Google Scholar]
- Kipke, D.R.; Vetter, R.J.; Williams, J.C.; Hetke, J.F. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 151–155. [Google Scholar]
- Normann, R.A.; Maynard, E.M.; Rousche, P.J.; Warren, D.J. A neural interface for a cortical vision prosthesis. Vision Res. 1999, 39, 2577–2587. [Google Scholar]
- Jones, K.E.; Campbell, P.K.; Normann, R.A. A glass silicon composite intracortical electrode array. Ann. Biomed. Eng. 1992, 20, 423–437. [Google Scholar]
- Campbell, P.K.; Jones, K.E.; Huber, R.J.; Horch, K.W.; Normann, R.A. A silicon-based, 3-Dimensional neural interface - manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 1991, 38, 758–768. [Google Scholar]
- Kelly, R.C.; Smith, M.A.; Samonds, J.M.; Kohn, A.; Bonds, A.B.; Movshon, J.A. Comparison of recordings from microelectrode arrays and single electrodes in the visual cortex. J. Neurosci. 2007, 27, 261–264. [Google Scholar]
- Nordhausen, C. T.; Rousche, P.J.; Normann, R. A. Chronic recordings of visually evoked responses using the utah intracortical electrode array. Eng. Med. Biol. Soc. 1993, 1391–1392. [Google Scholar]
- Rousche, P.J.; Normann, R.A. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J. Neurosci. Meth. 1998, 82, 1–15. [Google Scholar]
- Rousche, P.J.; Normann, R.A. Chronic intracortical microstimulation of cat auditory cortex using a 100 penetrating electrode array. J. Phys.-London. 1997, 499P, 87–88. [Google Scholar]
- Suner, S.; Fellows, M.R.; Vargas-Irwin, C.; Nakata, G.K.; Donoghue, J.P. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 524–541. [Google Scholar]
- Ensell, G.; Banks, D.J.; Richards, P.R.; Balachandran, W.; Ewins, D.J. Silicon-based microelectrodes for neurophysiology, micromachined from silicon-on-insulator wafers. Med. Biol. Eng. Comput. 2000, 38, 175–179. [Google Scholar]
- Norlin, P.; Kindlundh, M.; Mouroux, A.; Yoshida, K.; Hofmann, U.G. A 32-site neural recording probe fabricated by DRIE of SOI substrates. J. Micromech. Microeng. 2002, 12, 414–419. [Google Scholar]
- Kewley, D.T.; Hills, M.D.; Borkholder, D.A.; Opris, I.E.; Maluf, N.I.; Storment, C.W. Plasma-etched neural probes. Sens. Actuat. A-Phys. 1997, 58, 27–35. [Google Scholar]
- Cheung, K.; Gun, L.; Djupsund, K.; Yang, D.; Lee, L. P. A new neural probe using SOI wafers with topological interlocking mechanisms. IEEE Microtech. Med. Biol. 2000, 507–511. [Google Scholar]
- Kindlundh, M.; Norlin, P.; Hofmann, U.G. A neural probe process enabling variable electrode configurations. Sens. Actuat. B-Chem. 2004, 102, 51–58. [Google Scholar]
- Richardson, R.R.; Miller, J.A.; Reichert, W.M. Polyimides as biomaterials - preliminary biocompatibility testing. Biomaterials 1993, 14, 627–635. [Google Scholar]
- Rodriguez, F.J.; Ceballos, D.; Schuttler, M.; Valero, A.; Valderrama, E.; Stieglitz, T. Polyimide cuff electrodes for peripheral nerve stimulation. J. Neurosci. Meth. 2000, 98, 105–118. [Google Scholar]
- Seal, B.; Otero, T.; Panitch, A. Polymeric biomaterials for tissue and organ regeneration. Mater. Sci. Eng. 2001, 34, 147–230. [Google Scholar]
- Hetke, J. F.; Williams, J.C.; Pellinen, D. S.; Vetter, R. J.; Kipke, D. R. 3-D silicon probe array with hybrid polymer interconnect for chronic cortical recording. IEEE EMBS Conference on Neural Engineering, Capri Island, Italy; 2003. [Google Scholar]
- Subbaroyan, J.; Kipke, D.R. The role of flexible polymer interconnects in chronic tissue response induced by intracortical microelectrodes - a modeling and an in vivo study. IEEE EMBS Annu. Int. Conf., New York, USA; 2006. [Google Scholar]
- Takeuchi, S.; Suzuki, T.; Mabuchi, K.; Fujita, H. 3D flexible multichannel neural probe array. J. Micromech. Microeng. 2004, 14, 104–107. [Google Scholar]
- Lee, K.K.; He, J.P.; Singh, A.; Massia, S.; Ehteshami, G.; Kim, B. Polyimide-based intracortical neural implant with improved structural stiffness. J. Micromech. Microeng. 2004, 14, 32–37. [Google Scholar]
- Blum, N.A.; Carkhuff, B.G.; Charles, H.K.; Edwards, R.L.; Meyer, R.A. Multisite microprobes for neural recordings. IEEE Trans. Biomed. Eng. 1991, 38, 68–74. [Google Scholar]
- Szarowski, D.H.; Andersen, M.D.; Retterer, S.; Spence, A.J.; Isaacson, M.; Craighead, H.G. Brain responses to micro-machined silicon devices. Brain Res. 2003, 983, 23–35. [Google Scholar]
- Wood, N.K.; Kaminski, E.J.; Oglesby, R.J. The significance of implant shape is experimental testing of biological samples: disc vs rod. J. Biomed. Mat. Res. 1970, 4, 1–12. [Google Scholar]
- Taylor, S.R.; Gibbons, D.F. Effect of surface texture on the soft tissue response to polymer implants. J. Biomed. Mat. Res. 1983, 17, 205–227. [Google Scholar]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005, 195, 115–126. [Google Scholar]
- Burbaud, P.; Vital, A.; Rougier, A.; Bouillot, S.; Guehl, D.; Cuny, E. Minimal tissue damage after stimulation of the motor thalamus in a case of chorea-acanthocytosis. Neurology 2002, 59, 1982–1984. [Google Scholar]
- Britt, R.H.; Rossi, G.T. Quantitative-analysis of methods for reducing physiological brain pulsations. J. Neurosci. Meth. 1982, 6, 219–229. [Google Scholar]
- Fee, M.S. Active stabilization of electrodes for intracellular recording in awake behaving animals. Neuron. 2000, 27, 461–468. [Google Scholar]
- Spataro, L.; Dilgen, J.; Retterer, S.; Spence, A.J.; Isaacson, M.; Turner, J.N. Dexamethasone treatment reduces astroglia responses to inserted neuroprosthetic devices in rat neocortex. Exp. Neurol. 2005, 194, 289–300. [Google Scholar]
- Zhong, Y.H.; Bellamkonda, R.V. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007, 1148, 15–27. [Google Scholar]
- Zhong, Y.H.; Bellamkonda, R.V. Controlled release of anti-inflammatory agent alpha-MSH from neural implants. J. Control. Release 2005, 106, 309–18. [Google Scholar]
- Kennedy, P.R. The cone electrode - a long-term electrode that records from neurites grown onto its recording surface. J. Neurosci. Meth. 1989, 29, 181–93. [Google Scholar]
- Moxon, K.A.; Kalkhoran, N.M.; Markert, M.; Sambito, M.A.; McKenzie, J.L.; Webster, J.T. Nanostructured surface modification of ceramic-based microelectrodes to enhance biocompatibility for a direct brain-machine interface. IEEE Trans. Biomed. Eng. 2004, 51, 881–889. [Google Scholar]
- Sun, W.; Puzas, J.E.; Sheu, T.J.; Fauchet, P.M. Porous silicon as a cell interface for bone tissue engineering. Phys. Status Solidi A - Appl. Mat. Sci. 2007, 204, 1429–1433. [Google Scholar]
- Sun, W.; Puzas, J.E.; Sheu, T.J.; Fauchet, P.M. Nano-to-microscale porous silicon as a cell interface for bone tissue engineering. Adv. Mater. 2007, 19, 921–924. [Google Scholar]
- Thomas, V.; Dean, D.R.; Vohra, Y.K. Nanostructured biomaterials for regenerative medicine. Curr. Nanosci. 2006, 2, 155–177. [Google Scholar]














© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
HajjHassan, M.; Chodavarapu, V.; Musallam, S. NeuroMEMS: Neural Probe Microtechnologies. Sensors 2008, 8, 6704-6726. https://doi.org/10.3390/s8106704
HajjHassan M, Chodavarapu V, Musallam S. NeuroMEMS: Neural Probe Microtechnologies. Sensors. 2008; 8(10):6704-6726. https://doi.org/10.3390/s8106704
Chicago/Turabian StyleHajjHassan, Mohamad, Vamsy Chodavarapu, and Sam Musallam. 2008. "NeuroMEMS: Neural Probe Microtechnologies" Sensors 8, no. 10: 6704-6726. https://doi.org/10.3390/s8106704
APA StyleHajjHassan, M., Chodavarapu, V., & Musallam, S. (2008). NeuroMEMS: Neural Probe Microtechnologies. Sensors, 8(10), 6704-6726. https://doi.org/10.3390/s8106704