Pretargeted Imaging with Gallium-68—Improving the Binding Capability by Increasing the Number of Tetrazine Motifs
Abstract
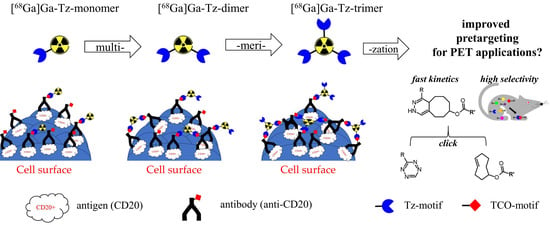
:1. Introduction
2. Results
2.1. (Radio) Chemistry
2.2. In Vitro Evaluation
2.3. In Vivo Evaluation
3. Discussion
4. Materials and Methods
4.1. Instrumentation
4.1.1. Analytical [radio]-RP-HPLC
4.1.2. Preparative RP-HPLC
4.1.3. MALDI-TOF MS
4.1.4. 1H-NMR Spectroscopy
4.2. Synthesis
4.2.1. General Information
4.2.2. [Fe]Fusarinine C ([Fe]FSC)
4.2.3. Acetylation of [Fe]FSC
4.2.4. Conjugation of Tetrazine-PEG5 Motif
4.2.5. Demetallation
- DAFC-PEG5-Tz (=Tz-monomer): 2.35 mg [1.81 µmol, 78%], gradient B (tR = 31.1 min); Analytical data: RP-HPLC tR = 11.5 min; MALDI TOF-MS: m/z [M + H]+ = 1303.95 [C60H89N11O21; exact mass: 1300.41 (calculated)]
- MAFC-(PEG5-Tz)2 (=Tz-dimer): 3.65 mg [2.09 µmol, 86%], gradient B (tR = 35.3 min); Analytical data: RP-HPLC tR = 12.5 min; MALDI TOF-MS: m/z [M + H]+ = 1748.25 [C81H118N16O27; exact mass: 1747.89 (calculated)]
- FSC-(PEG5-Tz)3 (=Tz-trimer): 1.68 mg [0.77 µmol, 60%], gradient B (tR = 37.9 min); Analytical data: RP-HPLC tR = 13.2 min; MALDI TOF-MS: m/z [M + H]+ = 2200.20 [C102H147N21O33; exact mass: 2195.38 (calculated)]
4.2.6. Modification of Rituximab (RTX)
4.3. Fluorescence Activated Cell-Sorting (FACS)
4.4. Radiolabelling of FSC-Based Tz-Conjugates with Gallium-68
4.5. In Vitro Characterization.
4.5.1. Distribution Coefficient (LogD)
4.5.2. Protein Binding
4.5.3. Stability Studies in Human Serum
4.5.4. Competitive Binding Assay
4.5.5. Cell Binding
4.6. In Vivo Characterization
4.6.1. Ethics Statement
4.6.2. Biodistribution Studies
4.6.3. Imaging Studies
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | acetonitrile |
| BSA | bovine serum albumin |
| CD | cluster of differentiation |
| DAFC | N,N′-diacetylfusarinine C |
| DIPEA | N,N-diisopropylamine |
| DMF | N,N-dimethylformamide |
| EDTA | ethylenediaminetetraacetic acid |
| FSC | fusarinine C |
| IEDDA | inverse electron-demand Diels-Alder |
| i.m. | intramuscular |
| mAb | monoclonal antibody |
| MAFC | N-monoacetylfusarinine C |
| NHS | N-hydroxysuccinimide |
| p.i. | post injection |
| PBS | phosphate buffered saline |
| PET/CT | positron emission computed tomography |
| r.o. | retro-orbitally |
| RP-HPLC | reversed phase high performance liquid chromatography |
| RT | room temperature |
| RTX | rituximab |
| TCO | trans-cyclooctene |
| TFA | trifluoracetic acid |
| Tz | tetrazine |
References
- Henricks, L.M.; Schellens, J.H.M.; Huitema, A.D.R.; Beijnen, J.H. The use of combinations of monoclonal antibodies in clinical oncology. Cancer Treat. Rev. 2015, 41, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, J. Current status and future directions of cancer immunotherapy. J. Cancer 2018, 9, 1773–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, S.M.; Wu, A.M. Advances in immuno-positron emission tomography: Antibodies for molecular imaging in oncology. J. Clin. Oncol. 2012, 30, 3884–3892. [Google Scholar] [CrossRef] [PubMed]
- Van De Watering, F.C.J.; Rijpkema, M.; Perk, L.; Brinkmann, U.; Oyen, W.J.G.; Boerman, O.C. Zirconium-89 labeled antibodies: A new tool for molecular imaging in cancer patients. Biomed. Res. Int. 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jauw, Y.W.S.; Menke-van der Houven van Oordt, C.W.; Hoekstra, O.S.; Hendrikse, N.H.; Vugts, D.J.; Zijlstra, J.M.; Huisman, M.C.; van Dongen, G.A.M.S. Immuno-positron emission tomography with zirconium-89-labeled monoclonal antibodies in oncology: What can we learn from initial clinical trials? Front. Pharmacol. 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Zarschler, K.; Pietzsch, H.-J.; Stephan, H.; Gasser, G. New insights into the pretargeting approach to image and treat tumours. Chem. Soc. Rev. 2016, 45, 6415–6431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailly, C.; Bodet-Milin, C.; Rousseau, C.; Faivre-Chauvet, A.; Kraeber-Bodéré, F.; Barbet, J. Pretargeting for imaging and therapy in oncological nuclear medicine. EJNMMI Radiopharm. Chem. 2017, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Altai, M.; Membreno, R.; Cook, B.; Tolmachev, V.; Zeglis, B.M. Pretargeted Imaging and Therapy. J. Nucl. Med. 2017, 58, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Karver, M.R.; Weissleder, R.; Hilderbrand, S.A. Synthesis and evaluation of a series of 1,2,4,5-tetrazines for bioorthogonal conjugation. Bioconjug. Chem. 2011, 22, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.L.; Guo, Z.; Bernardes, G.J.L. Inverse electron demand Diels–Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeglis, B.M.; Sevak, K.K.; Reiner, T.; Mohindra, P.; Carlin, S.D.; Zanzonico, P.; Weissleder, R.; Lewis, J.S. A Pretargeted PET Imaging Strategy Based on Bioorthogonal Diels–Alder Click Chemistry. J. Nucl. Med. 2013, 54, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.E.; Adumeau, P.; Membreno, R.; Carnazza, K.E.; Brand, C.; Reiner, T.; Agnew, B.J.; Lewis, J.S.; Zeglis, B.M. Pretargeted PET Imaging Using a Site-Specifically Labeled Immunoconjugate. Bioconjug. Chem. 2016, 27, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.P.; Houghton, J.L.; Kozlowski, P.; Abdel-Atti, D.; Reiner, T.; Pillarsetty, N.V.K.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. 18F-Based Pretargeted PET Imaging Based on Bioorthogonal Diels-Alder Click Chemistry. Bioconjug. Chem. 2016, 27, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Denk, C.; Svatunek, D.; Mairinger, S.; Stanek, J.; Filip, T.; Matscheko, D.; Kuntner, C.; Wanek, T.; Mikula, H. Design, Synthesis, and Evaluation of a Low-Molecular-Weight 11C-Labeled Tetrazine for Pretargeted PET Imaging Applying Bioorthogonal in Vivo Click Chemistry. Bioconjug. Chem. 2016, 27, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Zlitni, A.; Yin, M.; Janzen, N.; Chatterjee, S.; Lisok, A.; Gabrielson, K.L.; Nimmagadda, S.; Pomper, M.G.; Foster, F.S.; Valliant, J.F. Development of prostate specific membrane antigen targeted ultrasound microbubbles using bioorthogonal chemistry. PLoS ONE 2017, 12, e0176958. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.C.; Cornelissen, B. Bioorthogonal chemistry: Implications for pretargeted nuclear (PET/SPECT) imaging and therapy. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 96–113. [Google Scholar] [PubMed]
- Rossin, R.; Verkerk, P.R.; Van Den Bosch, S.M.; Vulders, R.C.M.; Verel, I.; Lub, J.; Robillard, M.S. In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem. Int. Ed. 2010, 49, 3375–3378. [Google Scholar] [CrossRef] [PubMed]
- Billaud, E.M.F.; Belderbos, S.; Cleeren, F.; Maes, W.; Van De Wouwer, M.; Koole, M.; Verbruggen, A.; Himmelreich, U.; Geukens, N.; Bormans, G. Pretargeted PET Imaging Using a Bioorthogonal 18F-Labeled trans-Cyclooctene in an Ovarian Carcinoma Model. Bioconjug. Chem. 2017, 28, 2915–2920. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, O.; Fung, K.; Pourat, J.; Jallinoja, V.; Vivier, D.; Pillarsetty, N.V.K.; Airaksinen, A.J.; Lewis, J.S.; Zeglis, B.M.; Sarparanta, M. Pretargeting of internalizing trastuzumab and cetuximab with a 18F-tetrazine tracer in xenograft models. EJNMMI Res. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.L.; Membreno, R.; Abdel-Atti, D.; Cunanan, K.M.; Carlin, S.; Scholz, W.W.; Zanzonico, P.B.; Lewis, J.S.; Zeglis, B.M. Establishment of the in vivo efficacy of pretargeted radioimmunotherapy utilizing inverse electron demand Diels- Alder click chemistry. Mol. Cancer Ther. 2017, 16, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Membreno, R.; Cook, B.E.; Fung, K.; Lewis, J.S.; Zeglis, B.M. Click-Mediated Pretargeted Radioimmunotherapy of Colorectal Carcinoma. Mol. Pharm. 2018, 15, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Läppchen, T.; Rossin, R.; van Mourik, T.R.; Gruntz, G.; Hoeben, F.J.M.; Versteegen, R.M.; Janssen, H.M.; Lub, J.; Robillard, M.S. DOTA-tetrazine probes with modified linkers for tumor pretargeting. Nucl. Med. Biol. 2017, 55, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.P.; Kozlowski, P.; Jackson, J.; Cunanan, K.M.; Adumeau, P.; Dilling, T.R.; Zeglis, B.M.; Lewis, J.S. Exploring Structural Parameters for Pretargeting Radioligand Optimization. J. Med. Chem. 2017, 60, 8201–8217. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.E.; Membreno, R.; Zeglis, B.M. A Dendrimer Scaffold for the Amplification of in Vivo Pretargeting Ligations. Bioconjug. Chem. 2018, 29, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, N.K.; Thurber, G.M.; Keliher, E.J.; Marinelli, B.; Weissleder, R. Reactive polymer enables efficient in vivo bioorthogonal chemistry. Proc. Natl. Acad. Sci. USA 2012, 109, 4762–4767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, B.; Qin, Z.; Yang, J.; Vera, D.R.; Devaraj, N.K. 68Ga chelating bioorthogonal tetrazine polymers for the multistep labeling of cancer biomarkers. Chem. Commun. 2014, 50, 5215–5217. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Summer, D.; Rangger, C.; Haas, H.; Haubner, R.; Decristoforo, C. Fusarinine C, a novel siderophore-based bifunctional chelator for radiolabeling with Gallium-68. J. Label. Compd. Radiopharm. 2015, 58, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summer, D.; Garousi, J.; Oroujeni, M.; Mitran, B.; Andersson, K.G.; Vorobyeva, A.; Löfblom, J.; Orlova, A.; Tolmachev, V.; Decristoforo, C. Cyclic versus Noncyclic Chelating Scaffold for 89Zr-Labeled ZEGFR:2377 Affibody Bioconjugates Targeting Epidermal Growth Factor Receptor Overexpression. Mol. Pharm. 2018, 15, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Knetsch, P.A.; Zhai, C.; Rangger, C.; Blatzer, M.; Haas, H.; Kaeopookum, P.; Haubner, R.; Decristoforo, C. [68Ga]FSC-(RGD)3 a trimeric RGD peptide for imaging αvβ3 integrin expression based on a novel siderophore derived chelating scaffold-synthesis and evaluation. Nucl. Med. Biol. 2015, 42, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Summer, D.; Rangger, C.; Franssen, G.M.; Laverman, P.; Haas, H.; Petrik, M.; Haubner, R.; Decristoforo, C. Novel Bifunctional Cyclic Chelator for 89Zr Labeling-Radiolabeling and Targeting Properties of RGD Conjugates. Mol. Pharm. 2015, 12, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Summer, D.; Rangger, C.; Klingler, M.; Laverman, P.; Franssen, G.M.; Lechner, B.E.; Orasch, T.; Haas, H.; von Guggenberg, E.; Decristoforo, C. Exploiting the concept of multivalency with 68Ga- and 89Zr-labelled Fusarinine C-minigastrin bioconjugates for targeting CCK2R expression. Contrast Media Mol. Imaging 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Summer, D.; Kroess, A.; Woerndle, R.; Rangger, C.; Klingler, M.; Haas, H.; Kremser, L.; Lindner, H.H.; Von Guggenberg, E.; Decristoforo, C. Multimerization results in formation of re- bindable metabolites: A proof of concept study with FSC-based minigastrin imaging probes targeting CCK2R expression. PLoS ONE 2018, 13, e0201224. [Google Scholar] [CrossRef] [PubMed]
- Rossin, R.; Lappchen, T.; van den Bosch, S.M.; Laforest, R.; Robillard, M.S. Diels-Alder Reaction for Tumor Pretargeting: In Vivo Chemistry Can Boost Tumor Radiation Dose Compared with Directly Labeled Antibody. J. Nucl. Med. 2013, 54, 1989–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, J.P.; Tully, K.M.; Jackson, J.; Dilling, T.R.; Reiner, T.; Lewis, J.S. Bioorthogonal Masking of Circulating Antibody-TCO Groups Using Tetrazine-Functionalized Dextran Polymers. Bioconjug. Chem. 2018, 29, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Rossin, R.; Van Duijnhoven, S.M.J.; Ten Hoeve, W.; Janssen, H.M.; Kleijn, L.H.J.; Hoeben, F.J.M.; Versteegen, R.M.; Robillard, M.S. Triggered Drug Release from an Antibody-Drug Conjugate Using Fast “click-to-Release” Chemistry in Mice. Bioconjug. Chem. 2016, 27, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Rossin, R.; Versteegen, R.M.; Wu, J.; Khasanov, A.; Wessels, H.J.; Steenbergen, E.J.; ten Hoeve, W.; Janssen, H.M.; van Onzen, A.H.A.M.; Hudson, P.J.; et al. Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumour activity in mice. Nat. Commun. 2018, 9, 1484. [Google Scholar] [CrossRef] [PubMed]
- Versteegen, R.M.; Wolter, T.; Rossin, R.; De Geus, M.A.; Janssen, H.M.; Robillard, M.S. Click-to-Release from trans-cyclooctenes: Mechanistic insights and expansion of scope from established carbamate to remarkable ether cleavage. Angew. Chem. Int. Ed. 2018, 57, 10494–10499. [Google Scholar] [CrossRef] [PubMed]
- Summer, D.; Grossrubatscher, L.; Petrik, M.; Michalcikova, T.; Novy, Z.; Rangger, C.; Klingler, M.; Haas, H.; Kaeopookum, P.; von Guggenberg, E.; et al. Developing Targeted Hybrid Imaging Probes by Chelator Scaffolding. Bioconjug. Chem. 2017, 28, 1722–1733. [Google Scholar] [CrossRef] [PubMed]






| FSC Subunit | Acetyl | PEG5-Tz Subunit | ||||||
|---|---|---|---|---|---|---|---|---|
| 3× CH | 3× CH3 | CH3 | Tetrazine | p-Phenylen | NH-CH2 | NH-CH2 | ||
| 6.3 ppm | 1.86 ppm | 1.83 ppm | 10.56 ppm | 8.44 ppm | 7.53 ppm | 8.50 ppm | 4.40 ppm | |
| Tz-monomer | 3 H | 9 H | 6 H | 1 H | 2 H | 2 H | 1 H | 2 H |
| Tz-dimer | 3 H | 9 H | 3 H | 2 H | 4 H | 4 H | 2 H | 4 H |
| Tz-trimer | 3 H | 9 H | none | 3 H | 6 H | 6 H | 3 H | 6 H |
| TAFC | 3 H | 9 H | 9 H | none | none | none | none | none |
| 68Ga-Labelled Compound | LogD (pH 7.4) | Protein Binding (%) | ||
|---|---|---|---|---|
| 1 h | 2 h | 4 h | ||
| Tz-monomer | −1.64 ± 0.02 | 61.8 ± 0.2 | 63.8 ± 2.1 | 64.0 ± 1.4 |
| Tz-dimer | −1.35 ± 0.01 | 67.0 ± 2.4 | 65.9 ± 1.3 | 68.4 ± 0.3 |
| Tz-trimer | −1.00 ± 0.06 | 70.5 ± 0.7 | 69.5 ± 0.4 | 67.8 ± 0.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Summer, D.; Mayr, S.; Petrik, M.; Rangger, C.; Schoeler, K.; Vieider, L.; Matuszczak, B.; Decristoforo, C. Pretargeted Imaging with Gallium-68—Improving the Binding Capability by Increasing the Number of Tetrazine Motifs. Pharmaceuticals 2018, 11, 102. https://doi.org/10.3390/ph11040102
Summer D, Mayr S, Petrik M, Rangger C, Schoeler K, Vieider L, Matuszczak B, Decristoforo C. Pretargeted Imaging with Gallium-68—Improving the Binding Capability by Increasing the Number of Tetrazine Motifs. Pharmaceuticals. 2018; 11(4):102. https://doi.org/10.3390/ph11040102
Chicago/Turabian StyleSummer, Dominik, Sonja Mayr, Milos Petrik, Christine Rangger, Katia Schoeler, Lisa Vieider, Barbara Matuszczak, and Clemens Decristoforo. 2018. "Pretargeted Imaging with Gallium-68—Improving the Binding Capability by Increasing the Number of Tetrazine Motifs" Pharmaceuticals 11, no. 4: 102. https://doi.org/10.3390/ph11040102
APA StyleSummer, D., Mayr, S., Petrik, M., Rangger, C., Schoeler, K., Vieider, L., Matuszczak, B., & Decristoforo, C. (2018). Pretargeted Imaging with Gallium-68—Improving the Binding Capability by Increasing the Number of Tetrazine Motifs. Pharmaceuticals, 11(4), 102. https://doi.org/10.3390/ph11040102