Design, Synthesis, Experimental and Theoretical Characterization of a New Multitarget 2-Thienyl-N-Acylhydrazone Derivative
Abstract
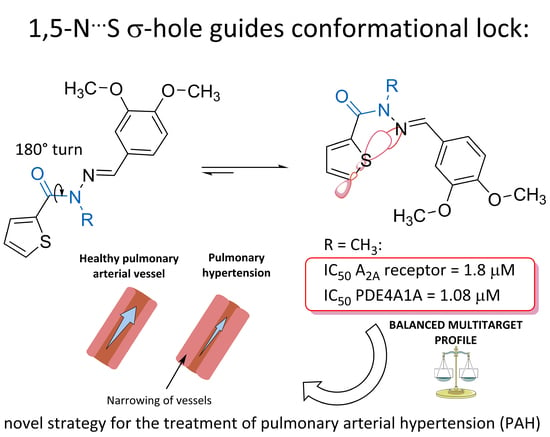
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Thienyl-N-Acylhydrazone Derivatives (3) and (4)
2.2. In Vitro Pharmacological Studies
2.3. Structure-Property Evaluation of Thienyl-N-Acylhydrazone Derivatives (3) and (4)
3. Experimental Section
3.1. Chemistry
3.2. In vitro Pharmacological Studies
3.3. Differential Scanning Calorimetry
3.4. X-ray Powder Diffraction
3.5. Indexing
3.6. Structure Determination and Rietveld Refinement
3.7. Molecular Modeling
3.8. Scanning Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fang, Z.; Song, Y.; Zhan, P.; Zhang, Q.; Liu, X. Conformational restriction: An effective tactic in ’follow-on’-based drug discovery. Future Med. Chem. 2014, 6, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.J.; Fraga, C.A.M. Química Medicinal. As Bases Moleculares da Ação dos Fármacos, 3rd ed.; Atrmed: Porto Alegre, Brazil, 2015; ISBN 9788582711187. [Google Scholar]
- Wermuth, C.G. The Practice of Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780123741943. [Google Scholar]
- Kümmerle, A.E.; Raimundo, J.M.; Leal, C.M.; da Silva, G.S.; Balliano, T.L.; Pereira, M.A.; de Simone, C.A.; Sudo, R.T.; Zapata-Sudo, G.; Fraga, C.A.M. Studies towards the identification of putative bioactive conformation of potent vasodilator arylidene N-acylhydrazone derivatives. Eur. J. Med. Chem. 2009, 44, 4004–4009. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.J.; Kümmerle, A.E.; Fraga, C.A.M. The Methylation Effect in Medicinal Chemistry. Chem. Rev. 2011, 111, 5215–5246. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.d.S.M.; Rodrigues, D.A.; Alves, M.A.; Tinoco, L.W.; Ferreira, G.B.; de Sant’Anna, C.M.R.; Fraga, C.A.M. Theoretical and experimental characterization of 1,4-N⋯S σ-hole intramolecular interactions in bioactive N -acylhydrazone derivatives. New J. Chem. 2018, 42, 497–505. [Google Scholar] [CrossRef]
- Beno, B.R.; Yeung, K.-S.; Bartberger, M.D.; Pennington, L.D.; Meanwell, N.A. A Survey of the Role of Noncovalent Sulfur Interactions in Drug Design. J. Med. Chem. 2015, 58, 4383–4438. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Politzer, P. Simultaneous σ-hole and hydrogen bonding by sulfur- and selenium-containing heterocycles. Int. J. Quantum Chem. 2008, 108, 2770–2781. [Google Scholar] [CrossRef]
- Da Rocha, M.D. Novos protótipos heteroaril-N-acilidrazônicos planejados para o tratamento da hipertensão arterial pulmonary; Federal University of Rio de Janeiro: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Alencar, A.K.N.; Montes, G.C.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. Adenosine Receptors as Drug Targets for Treatment of Pulmonary Arterial Hypertension. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A.M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.; Barreiro, E.J. Beyond Bioisosterism: New Concepts in Drug Discovery. In Comprehensive Medicinal Chemistry III; Elsevier: Amsterdam, The Netherlands, 2017; pp. 186–210. [Google Scholar]
- Lima, L.; Barreiro, E. Bioisosterism: A Useful Strategy for Molecular Modification and Drug Design. Curr. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.M.; Pereira, S.L.; Kümmerle, A.E.; Leal, D.M.; Tesch, R.; De Sant’Anna, C.M.R.; Fraga, C.A.M.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. Antihypertensive profile of 2-thienyl-3,4-methylenedioxybenzoylhydrazone is mediated by activation of the A2A adenosine receptor. Eur. J. Med. Chem. 2012, 55, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kümmerle, A.E.; Schmitt, M.; Cardozo, S.V.S.; Lugnier, C.; Villa, P.; Lopes, A.B.; Romeiro, N.C.; Justiniano, H.; Martins, M.A.; Fraga, C.A.M.; et al. Design, Synthesis, and Pharmacological Evaluation of N -Acylhydrazones and Novel Conformationally Constrained Compounds as Selective and Potent Orally Active Phosphodiesterase-4 Inhibitors. J. Med. Chem. 2012, 55, 7525–7545. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, R.B.; da Silva, L.L.; de Lima, C.K.F.; Miguez, E.; Miranda, A.L.P.; Laufer, S.A.; Barreiro, E.J.; Fraga, C.A.M. Discovery of Novel Orally Active Anti-Inflammatory N-Phenylpyrazolyl-N-Glycinyl-Hydrazone Derivatives That Inhibit TNF-α Production. PLoS ONE 2012, 7, e46925. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Miguez, E.; Kümmerle, A.; Rumjanek, V.; Fraga, C.; Barreiro, E. Characterization of Amide Bond Conformers for a Novel Heterocyclic Template of N-acylhydrazone Derivatives. Molecules 2013, 18, 11683–11704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di, L.; Fish, P.V.; Mano, T. Bridging solubility between drug discovery and development. Drug Discov. Today 2012, 17, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Moura, V.J.G.; Alencar, A.K.N.; de Albuquerque Calasans-Maia, J.; da Silva, J.S.; Fraga, C.A.M.; Zapata-Sudo, G.; Barreiro, E.J.; Sudo, R.T. Novel Agonist of Adenosine Receptor Induces Relaxation of Corpus Cavernosum in Guinea Pigs: An In Vitro and In Vivo Study. Urology 2015, 85, 1214.e17–1214.e21. [Google Scholar] [CrossRef] [PubMed]
- Luthin, D.R.; Linden, J. Comparison of A4 and A2a binding sites in striatum and COS cells transfected with adenosine A2a receptors. J. Pharmacol. Exp. Ther. 1995, 272, 511–518. [Google Scholar] [PubMed]
- Coelho, A.A.; Evans, J.; Evans, I.; Kern, A.; Parsons, S. The TOPAS symbolic computation system. Powder Diffr. 2011, 26, S22–S25. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 1981, 14, 357–361. [Google Scholar] [CrossRef] [Green Version]
- David, W.I.F.; Shankland, K.; Van De Streek, J.; Pidcock, E.; Motherwell, W.D.S.; Cole, J.C. DASH: A program for crystal structure determination from powder diffraction data. J. Appl. Crystallogr. 2006, 39, 910–915. [Google Scholar] [CrossRef]
- Bastos, I.T.S.; Costa, F.N.; Silva, T.F.; Barreiro, E.J.; Lima, L.M.; Braz, D.; Lombardo, G.M.; Punzo, F.; Ferreira, F.F.; Barroso, R.C. A combined experimental and in silico characterization to highlight additional structural features and properties of a potentially new drug. J. Mol. Struct. 2017, 1146, 735–743. [Google Scholar] [CrossRef]
- Ferreira, F.F.; Trindade, A.C.; Antonio, S.G.; de Oliveira Paiva-Santos, C. Crystal structure of propylthiouracil determined using high-resolution synchrotron X-ray powder diffraction. CrystEngComm 2011, 13, 5474. [Google Scholar] [CrossRef]
- Ferreira, F.F.; Antonio, S.G.; Rosa, P.C.P.; de Oliveira Paiva-Santos, C. Crystal structure determination of mebendazole form A using high-resolution synchrotron x-ray powder diffraction data. J. Pharm. Sci. 2010, 99, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Cheary, R.W.; Coelho, A.A. Axial Divergence in a Conventional X-ray Powder Diffractometer. I. Theoretical Foundations. J. Appl. Crystallogr. 1998, 31, 851–861. [Google Scholar] [CrossRef] [Green Version]
- Cheary, R.W.; Coelho, A.A. Axial Divergence in a Conventional X-ray Powder Diffractometer. II. Realization and Evaluation in a Fundamental-Parameter Profile Fitting Procedure. J. Appl. Crystallogr. 1998, 31, 862–868. [Google Scholar] [CrossRef]
- Balzar, D. X-ray diffraction line broadening: Modeling and applications to high-Tc superconductors. J. Res. Natl. Inst. Stand. Technol. 1993, 98, 321. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, M. Application of symmetrized harmonics expansion to correction of the preferred orientation effect. J. Appl. Crystallogr. 1993, 26, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Natural bond orbitals and extensions of localized bonding concepts. Chem. Educ. Res. Pr. 2001, 2, 91–104. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]









 | ||||
|---|---|---|---|---|
| Compounds | PDE4A1A a | PDE4B1 a | PDE4C a | PDE4D3 a |
| R = H (3) | 53% | 48% | 12% | 19% |
| R = CH3 (4) | 78% | 44% | 22% | 40% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos, I.T.S.; Pinheiro, P.d.S.M.; Costa, F.N.; Rocha, M.D.; Sant’Anna, C.M.R.; Braz, D.; Souza, E.T.; Martins, M.A.; Barreiro, E.J.; Ferreira, F.F.; et al. Design, Synthesis, Experimental and Theoretical Characterization of a New Multitarget 2-Thienyl-N-Acylhydrazone Derivative. Pharmaceuticals 2018, 11, 119. https://doi.org/10.3390/ph11040119
Bastos ITS, Pinheiro PdSM, Costa FN, Rocha MD, Sant’Anna CMR, Braz D, Souza ET, Martins MA, Barreiro EJ, Ferreira FF, et al. Design, Synthesis, Experimental and Theoretical Characterization of a New Multitarget 2-Thienyl-N-Acylhydrazone Derivative. Pharmaceuticals. 2018; 11(4):119. https://doi.org/10.3390/ph11040119
Chicago/Turabian StyleBastos, Isadora T. S., Pedro de Sena M. Pinheiro, Fanny N. Costa, Miguel D. Rocha, Carlos Mauricio R. Sant’Anna, Delson Braz, Everton T. Souza, Marco A. Martins, Eliezer J. Barreiro, Fabio F. Ferreira, and et al. 2018. "Design, Synthesis, Experimental and Theoretical Characterization of a New Multitarget 2-Thienyl-N-Acylhydrazone Derivative" Pharmaceuticals 11, no. 4: 119. https://doi.org/10.3390/ph11040119
APA StyleBastos, I. T. S., Pinheiro, P. d. S. M., Costa, F. N., Rocha, M. D., Sant’Anna, C. M. R., Braz, D., Souza, E. T., Martins, M. A., Barreiro, E. J., Ferreira, F. F., Barroso, R. C., & Fraga, C. A. M. (2018). Design, Synthesis, Experimental and Theoretical Characterization of a New Multitarget 2-Thienyl-N-Acylhydrazone Derivative. Pharmaceuticals, 11(4), 119. https://doi.org/10.3390/ph11040119