Troxerutin Prevents 5-Fluorouracil Induced Morphological Changes in the Intestinal Mucosa: Role of Cyclooxygenase-2 Pathway
Abstract
:1. Introduction
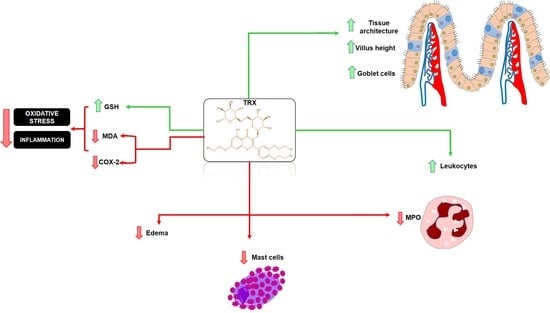
2. Results
2.1. Characterization of Troxerutin
2.2. Weight Analysis
2.3. Histopathological and Morphometric Analysis
2.4. Leukocyte Count
2.5. Myeloperoxidase Assay (MPO)
2.6. Malondialdehyde (MDA) and Glutathione (GSH) levels
2.7. Cell Count of the Intestinal Mucosa: Mast and Goblet Cells
2.8. Effect of TRX on Cyclooxygenase-2 Pathway Based on Histopathological and Morphometric Analyses
2.9. Immunohistochemistry for the Detection of COX-2
2.10. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. TRX Synthesis
4.2. TRX Characterization
4.3. Drugs and Reagents
4.4. Animals
4.5. Experimental Protocol of 5-FU-Induced Intestinal Mucositis
4.6. White Blood Cell Count
4.7. Histopathological and Morphometric Analysis
4.8. Myeloperoxidase Assay (MPO)
4.9. Measurement of GSH and MDA Levels
4.10. Intestinal Mucosa Cell Count: Goblet and Mast Cells
4.11. Immunohistochemistry for the Detection of COX-2
4.12. Molecular Docking and Determination of TRX Binding Sites
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medeiros, A.D.C.; Azevedo, Í.M.; Lima, M.L.; Araújo-Filho, I.; Moreira, M.D. Effects of simvastatin on 5-fluorouracil-induced gastrointestinal mucositis in rats. Rev. Col. Bras. Cir. 2018, 45, e1968. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.J.; Keefe, D.M.; Lalla, R.V.; Bateman, E.; Blijlevens, N.; Fijlstra, M.; Fijlstra, M.; King, E.E.; Stringer, A.M.; Van der Velden, W.J.F.M.; et al. Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Support. Care Cancer 2013, 21, 313–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, D.E.; Bensadoun, R.J.; Roila, F. ESMO Guidelines Working Group. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann. Oncol. 2011, 22, 78–84. [Google Scholar] [CrossRef] [PubMed]
- D’Hondt Lionel, L.C.; Marc, A.; Jean-Luc, C. Oral mucositis induced by anticancer treatments: Physiopathology and treatments. Ther. Clin. Risk Manag. 2006, 2, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Keefe, D.M. Intestinal mucositis: Mechanisms and management. Curr. Opin. Oncol. 2007, 19, 323–327. [Google Scholar] [CrossRef]
- Mercadante, S.; Aielli, F.; Adile, C.; Ferrera, P.; Valle, A.; Fusco, F.; Caruselli, A.; Cartoni, C.; Massimo, P.; Masedu, F.; et al. Prevalence of oral mucositis, dry mouth, and dysphagia in advanced cancer patients. Support. Care Cancer 2015, 23, 3249–3255. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Moon, W.; Park, J.; Park, S.J.; Am Song, G.; Han, S.H.; Lee, J.H. Rebamipide attenuates 5-fluorouracil-induced small intestinal mucositis in a mouse model. Biol. Pharm. Bull. 2015, 38, 179–183. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, A.A.; Borba, P.B.; De Souza, F.H.D.; Nogueira, A.C.; Saldanha, T.S.; Araújo, T.E.F.; Da Silva, A.I.; De Araújo Júnior, R.F. In a methotrexate-induced model of intestinal mucositis, olmesartan reduced inflammation and induced enteropathy characterized by severe diarrhea, weight loss, and reduced sucrose activity. Biol. Pharm. Bull. 2015, 38, 746–752. [Google Scholar] [CrossRef] [Green Version]
- Peterson, D.E. Oral and gastrointestinal mucositis: Novel insights into pathophysiology and potential therapies. Adv. Stud. Med. 2005, 5, S299–S310. [Google Scholar]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Guabiraba, R.; Besnard, A.G.; Menezes, G.B.; Secher, T.; Jabir, M.S.; Amaral, S.S.; Braun, H.; Lima-Junior, R.C.; Ribeiro, R.A.; Cunha, F.Q.; et al. IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. Mucosal Immunol. 2014, 7, 1079–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, R.S.; Barros, A.L.B. Intestinal Mucositis Induced by Chemotherapy: Na Oviervew. J. Mol. Pharm. Org. Process Res. 2015, 3, e123. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, R.M.; Sanfilippo, N.; Paster, B.J.; Kerr, A.R.; Li, Y.; Ramalho, L.; Queiroz, E.L.; Smith, B.; Sonis, S.T.; Corby, P.M. Host-microbiome cross-talk in oral mucositis. J. Dent. Res. 2016, 95, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Mancini, M.; Sonis, S.T.; Fernandez-Martinez, J.; Liu, J.; Cohen, E.E.; Toback, F.G. A novel peptide for simultaneously enhanced treatment of head and neck cancer and mitigation of oral mucositis. PLoS ONE 2016, 11, e0152995. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, B.A.; Rao, M.U.; Muhammad, A.; Zin, T.; Mohamad, N.H.; Mohamad, N.; Mohd, K.S. Reviews of herbal and their secondary metabolites in the treatment of ulcerative colitis and peptic ulcer. J. Appl. Pharm. Sci. 2014, 4, 080–090. [Google Scholar] [CrossRef]
- Bahmani, M.; Zargaran, A.; RafieiaN-Kopaei, M. Identification of medicinal plants of Urmia for treatment of gastrointestinal disorders. Rev. Bras. Farmacogn. 2014, 24, 468–480. [Google Scholar] [CrossRef] [Green Version]
- Shan, Q.; Zhuang, J.; Zheng, G.; Zhang, Z.; Zhang, Y.; Lu, J.; Zheng, Y. Troxerutin Reduces Kidney damage against BDE-47-induced apoptosis via inhibiting NOX2 activity and increasing Nrf2 activity. Oxid. Med. Cell Longev. 2017, 2017, e6034692. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.S.; George, K.; Selvam, A.A.A. Troxerutin subdues hepatic tumorigenesis via disrupting the MDM2–p53 interaction. Food Funct. 2018, 9, 5336–5349. [Google Scholar] [CrossRef]
- Dehnamaki, F.; Karimi, A.; Pilevarian, A.A.; Fatemi, I.; Hakimizadeh, E.; Kaeidi, A.; Allahtavakoli, M.; Rahmani, M.R.; Khademalhosseini, M.; Bazmandegan, G. Treatment with troxerutin protects against cisplatin-induced kidney injury in mice. Acta Chir. Belg. 2018, 119, 31–37. [Google Scholar] [CrossRef]
- Shan, Q.; Zheng, G.H.; Han, X.R.; Wen, X.; Wang, S.; Li, M.Q.; Zheng, Y.L. Troxerutin protects kidney tissue against BDE-47-induced inflammatory damage through CXCR4-TXNIP/NLRP3 signaling. Oxid. Med. Cell. Longev. 2018, 2018, e9865495. [Google Scholar] [CrossRef] [Green Version]
- Geetha, R.; Priya, C.S.; Anuradha, C.V. Troxerutin attenuates diet-induced oxidative stress, impairment of mitochondrial biogenesis and respiratory chain complexes in mice heart. Clin. Exp. Pharmacol. Physiol. 2017, 44, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Baluchnejadmojarad, T.; Jamali-Raeufy, N.; Zabihnejad, S.; Rabiee, N.; Roghani, M. Troxerutin exerts neuroprotection in 6-hydroxydopamine lesion rat model of Parkinson’s disease: Possible involvement of PI3K/ERβ signaling. Eur. J. Pharmacol. 2017, 801, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ngeow, W.C.; Chai, W.L.; Zain, R.B. Management of radiation therapy-induced mucositis in head and neck cancer patients. Part II: Supportive treatments. Oncol. Rev. 2008, 2, 164–182. [Google Scholar] [CrossRef]
- Muri, E.M.; Sposito, M.M.M.; Metsavaht, L. Pharmacology of vasoactive drugs. Acta Fisiatr. 2010, 17, 22–27. [Google Scholar]
- Gui, Y.; Li, A.; Chen, F.; Zhou, H.; Tang, Y.; Chen, L.; Duan, S. Involvement of AMPK/SIRT1 pathway in anti-allodynic effect of troxerutin in CCI-induced neuropathic pain. Eur. J. Pharmacol. 2015, 769, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Satinsky, D.; Jägerová, K.; Havlíková, L.; Solich, P. A new and fast HPLC method for determination of rutin, troxerutin, diosmin and hesperidin in food supplements using fused-core column technology. Food Anal. Methods 2013, 6, 1353–1360. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Mao, P.; Zhao, Z.; Yang, L.R.; Lin, X.F. Regioselective enzymatic acylation of troxerutin in non aqueous medium. Chin. Chem. Lett. 2010, 21, 59–62. [Google Scholar] [CrossRef]
- Xu, J.D.; Zhang, L.W.; Liu, Y.F. Synthesis and antioxidant activities of flavonoids derivatives, troxerutin and 3′,4′,7-triacetoxyethoxy quercetin. Chin. Chem. Lett. 2013, 24, 223–226. [Google Scholar] [CrossRef]
- Liu, Z.; Xi, J.; Schröder, S.; Wang, W.; Xie, T.; Wang, Z.; Bao, S.; Fei, J. Chimonanthus nitens var. salicifolius aqueous extract protects against 5-fluorouracil induced gastrointestinal mucositis in a mouse model. Evid. Based Complement. Altern. Med. 2013, 2013, 789263. [Google Scholar] [CrossRef]
- Chen, X.X.; Lam, K.H.; Chen, Q.X.; Leung, G.P.H.; Tang, S.C.W.; Sze, S.C.W.; Zhang, Z.J. Ficus virens proanthocyanidins induced apoptosis in breast cancer cells concomitantly ameliorated 5-fluorouracil induced intestinal mucositis in rats. Food Chem. Toxicol. 2017, 110, 49–61. [Google Scholar] [CrossRef]
- Cheah, K.Y.; Howarth, G.S.; Yazbeck, R.; Wright, T.H.; Whitford, E.J.; Payne, C.; Bastian, S. Grape seed extract protects IEC-6 cells from chemotherapy-induced cytotoxicity and improves parameters of small intestinal mucositis in rats with experimentally-induced mucositis. Cancer Biol. Ther. 2009, 8, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cechinel-zanchett, C.C.; Boeing, T.; Somensi, L.B.; Steimbach, V.M.B.; Campos, A.; Krueger, C.D.M.A.; Faloni de Andrade, S. Flavonoid-rich fraction of Bauhinia forficata Link leaves prevents the intestinal toxic effects of irinotecan chemotherapy in IEC-6 cells and in mice. Phytother. Res. 2019, 33, 90–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.K.; Park, M.Y.; Sung, M.K. 5-Fluorouracil-induced changes of intestinal integrity biomarkers in BALB/c mice. J. Cancer Prev. 2013, 18, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, P.M.; Mota, J.M.S.; Gomes, A.S.; Oliveira, R.B.; Assreuy, A.M.S.; Brito, G.A.C.; Souza, M.H. Gastrointestinal dysmotility in 5-fluorouracil-induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemother. Pharm. 2008, 63, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.M.G.; Mota, J.M.S.C.; Souza, E.P.; Justino, P.F.C.; Franco, A.X.; Cunha, F.Q.; Ribeiro, R.A.; Souza, M.H.L.P. Inflammatory intestinal damage induced by 5-fluorouracil requires IL-4. Cytokine 2013, 61, 46–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Generoso, S.V.; Rodrigues, N.M.; Trindade, L.M.; Paiva, N.C.; Cardoso, V.N.; Carneiro, C.M.; Maioli, T.U. Dietary supplementation with omega-3 fatty acid attenuates 5-fluorouracil induced mucositis in mice. Lipids Health Dis. 2015, 14, 54. [Google Scholar] [CrossRef] [Green Version]
- Galdino, F.M.P.; Andrade, M.E.R.; De Barros, P.A.V.; Generoso, S.V.; Leite, J.I.A.; De Almeida-Leite, C.M.; Peluzio, M.C.G.; Fernandes, S.O.A.; Cardoso, V.N. Pretreatment and treatment with fructo-oligosaccharides attenuate intestinal mucositis induced by 5-FU in mice. J. Funct. Foods 2018, 49, 485–492. [Google Scholar] [CrossRef]
- Costa, D.V.S.; Bon-Frauches, A.C.; Silva, A.M.H.P.; Lima-Júnior, R.C.P.; Martins, C.S.; Leitão, R.F.C.; Freitas, G.B.; Castelucci, P.; Bolick, D.T.; Guerrant, R.L.; et al. 5-fluorouracil induces enteric neuron death and glial activation during intestinal mucositis via a S100B-RAGE-NFκB-dependent pathway. Sci. Rep. 2019, 9, 665. [Google Scholar] [CrossRef] [Green Version]
- Miranda, J.A.L.; Barreto, J.E.F.; Martins, D.S.; De Souza Pimentel, P.V.; Da Silva Costa, D.V.; Silva, R.R.; Souza, L.K.M.; Lima, C.N.C.; Rocha, J.A.; Freitas, A.P.F.; et al. Protective effect of cashew gum (Anacardium occidentale L.) on 5-fluorouracil-induced intestinal mucositis. Pharmaceuticals 2019, 12, 51. [Google Scholar] [CrossRef] [Green Version]
- Sonis, S.T. A biological approach to mucositis. J. Support. Oncol. 2004, 2, 21–36. [Google Scholar]
- Kumar, S.; Aninat, C.; Michaux, G.; Morel, F. Anticancer drug 5-fluorouracil induces reproductive and developmental defects in Caenorhabditis elegans. Reprod. Toxicol. 2010, 29, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Kobuchi, S.; Ito, Y.; Sakaeda, T. Population pharmacokinetic–pharmacodynamic modeling of 5-fluorouracil for toxicities in rats. Eur. J. Drug Metab. Pharm. 2017, 42, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Monteseirín, J.; Bonilla, I.; Camacho, J.; Conde, J.; Sobrino, F. Elevated secretion of myeloperoxidase by neutrophils from asthmatic patients: The effect of immunotherapy. J. Allergy Clin. Immunol. 2001, 107, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Lacy, P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.L.; Hong, H.; Monfregola, J.; Catz, S.D. Increased survival and reduced neutrophil infiltration of the liver in Rab27a- but not Munc13-4-deficient mice in lipopolysaccharide-induced systemic inflammation. Infect. Immun. 2011, 79, 3607–3618. [Google Scholar] [CrossRef] [Green Version]
- Rymaszewski, A.L.; Tate, E.; Yimbesalu, J.; Gelman, A.; Jarzembowski, J.; Zhang, H.; Vikis, H. The role of neutrophil myeloperoxidase in models of lung tumor development. Cancers 2014, 6, 1111–1127. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.D. Pharmacological activities of flavonoids: A review. Int. J. Pharm. Sci. Nanotech. 2011, 4, 1394–1398. [Google Scholar]
- Sangeetha, K.S.S.; Umamaheswari, S.; Reddy, C.U.M.; Kalkura, S.N. Flavonoids: Therapeutic potential of natural pharmacological agents. Int. J. Pharm. Sci. Res. 2016, 7, 3924–3930. [Google Scholar] [CrossRef]
- Hayat, M.; Abbas, M.; Munir, F.; Hayat, M.Q.; Keyani, R.; Amir, R. Potential of plant flavonoids in pharmaceutics and nutraceutics. J. Biomol. Biochem. 2017, 1, 12–17. [Google Scholar]
- Wallace, J.L. Recent advances in gastric ulcer therapeutics. Curr. Opin. Pharmacol. 2005, 5, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, D.B.; Stephen, S.; Borum, M.; Voltaggio, L.; Doman, D.B. Mast cells in gastrointestinal disease. Gastroenterol. Hepatol. 2010, 6, 772–777. [Google Scholar]
- Kheirollahi, A.; Abbaszadeh, A.; Anbari, K.; Rostami, B.; Ahangari, A.; Hasanvand, A.; Gholami, M. Troxerutin protect sperm, seminiferous epithelium and pituitary-gonadal axis from torsion-detorsion injury: An experimental study. Int. J. Reprod. Biomed. 2018, 16, 315–322. [Google Scholar] [CrossRef]
- Gibson, R.J.; Bowen, J.M.; Keefe, D.M.K. Technological advances in mucositis research: New insights and new issues. Cancer Treat. Rev. 2008, 34, 476–482. [Google Scholar] [CrossRef]
- Gehart, H.; Clevers, H. Tales from the crypt: New insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 2018, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Khalili, J.; Biloklytska, H.F. Salivary malondialdehyde levels in clinically healthy and periodontal diseased individuals. Oral Dis. 2008, 14, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Vinothkumar, R.; Kumar, R.V.; Karthikkumar, V.; Viswanathan, P.; Kabalimoorthy, J.; Nalini, N. Oral supplementation with troxerutin (trihydroxyethylrutin), modulates lipid peroxidation and antioxidant status in 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Environ. Toxicol. Pharmacol. 2014, 37, 174–184. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Fan, S.H.; Zheng, Y.L.; Lu, J.; Wu, D.M.; Shan, Q.; Hu, B. Troxerutin improves hepatic lipid homeostasis by restoring NAD+ depletion-mediated dysfunction of lipin 1 signaling in high-fat diet-treated mice. Biochem. Pharmacol. 2014, 91, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Badalzadeh, R.; Layeghzadeh, N.; Alihemmati, A.; Mohammadi, M. Beneficial effect of troxerutin on diabetes-induced vascular damages in rat aorta: Histopathological alterations and antioxidation mechanism. Int. J. Endocrinol. Metab. 2015, 13, e25969. [Google Scholar] [CrossRef] [Green Version]
- Geetha, R.; Priya, C.S.; Anuradha, C.V. Troxerutin abrogates mitochondrial oxidative stress and myocardial apoptosis in mice fed calorie-rich diet. Chem. Biol. Interact. 2017, 278, 74–83. [Google Scholar] [CrossRef]
- Thomas, N.S.; George, K.; Arivalagan, S.; Mani, V.; Siddique, A.I.; Namasivayam, N. The in vivo antineoplastic and therapeutic efficacy of troxerutin on rat preneoplastic liver: Biochemical, histological and cellular aspects. Eur. J. Nutr. 2016, 56, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J. Neuropathol. Exp. Neurol. 2004, 63, 901–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouzer, C.A.; Marnett, L.J. Cyclooxygenases: Structural and functional insights. J. Lipid Res. 2009, 50, S29–S34. [Google Scholar] [CrossRef] [Green Version]
- Clark, M.A. Fármacos antiinflamatórios. In Farmacologia Ilustrada, 5th ed.; Clark, M.A., Finkel, R., Rey, J.A., Whalen, K., Eds.; Artmed: Porto Alegre, Brazil, 2013; pp. 525–548. [Google Scholar]
- Yeoh, A.S.; Gibson, R.J.; Yeoh, E.E.; Bowen, J.M.; Stringer, A.M.; Giam, K.A.; Keefe, D.M. A novel animal model to investigate fractionated radiotherapy-induced alimentary mucositis: The role of apoptosis, p53, nuclear factor-κB, COX-1, and COX-2. Mol. Cancer Ther. 2007, 6, 2319–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, D.V.; Lima-Júnior, R.C.; Carvalho, C.B.; Borges, V.F.; Wanderley, C.W.; Bem, A.X.; Cunha, T.M. The adaptor protein Myd88 is a key signaling molecule in the pathogenesis of irinotecan-induced intestinal mucositis. PLoS ONE 2015, 10, e0139985. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Ranjan, R.; Singh, A.; Yashavarddhan, M.H.; Bajaj, S.; Gupta, M.L. A combination of podophyllotoxin and rutin attenuates radiation induced gastrointestinal injury by negatively regulating NF-κB/p53 signaling in lethally irradiated mice. PLoS ONE 2016, 11, e0168525. [Google Scholar] [CrossRef]
- Alencar, N.M.N.; Da Silveira Bitencourt, F.; De Figueiredo, I.S.T.; Luz, P.B.; Lima-Júnior, R.C.P.; Aragão, K.S.; Ramos, M.V. Side-effects of Irinotecan (CPT-11), the clinically used drug for colon cancer therapy, are eliminated in experimental animals treated with latex proteins from Calotropis procera (Apocynaceae). Phytother. Res. 2017, 31, 312–320. [Google Scholar] [CrossRef]
- Courbat, P.; Favre, J.; Guerven, R.; Uhlmann, G. Contribution a L’edude D’unprodut de β-hidroxyethylationdurutoside Parte 1. Helv. Chim. Acta 1966, 49, 1203–1211. [Google Scholar] [CrossRef]
- Courbat, P.; Uhlmann, G.; Guerven, R. Contribution a L’etude D’unProdult de β-hidroxyethylationdurutoside Parte 2. Helv. Chim. Acta 1966, 49, 1420–1424. [Google Scholar] [CrossRef]
- Moura, R.; Wada, C.; Purchio, A.; Almeida, T. Studies of the Figurative Elements of Blood, 3rd ed.; Atheneu: São Paulo, Brazil, 1998. [Google Scholar]
- Dos Santos Filho, E.X.; Ávila, P.H.M.; Bastos, C.C.C.; Batista, A.C.; Naves, L.N.; Marreto, R.N.; Lima, E.M.; Mendonca, E.F.; Valadares, M.C. Curcuminoids from Curcuma longa L. reduced intestinal mucositis induced by 5-fluorouracil in mice: Bioadhesive, proliferative, anti-inflammatory and antioxidant effects. Toxicol. Rep. 2016, 3, 55–62. [Google Scholar] [CrossRef] [Green Version]
- MacPherson, B.; Pfeiffer, C. Experimental production of diffuse colitis in rats. Digestion 1978, 17, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Michalany, J. Histological Technique Pathological Anatomy: With Instructions for the Surgeon, Nurse, Cytotechnician, 3rd ed.; Michalany: São Paulo, Brazil, 2008. [Google Scholar]
- Sano, T.; Utsumi, D.; Amagase, K.; Matsumoto, K.; Tominaga, M.; Higuchi, K.; Takeuchi, T.; Kato, T. Lafutidine, a histamine h2 receptor antagonist with mucosal protective properties, attenuates 5-fluorouracil-induced intestinal mucositis in mice through activation of extrinsic primaryafferent neurons. J. Physiol. Pharmacol. 2017, 68, 79–90. [Google Scholar]
- Goodsell, D.S.; Morris, G.M.; Olson, A.J. Automated docking of flexible ligands: Applications of autodock. J. Mol. Recognit. 1996, 9, 1–5. [Google Scholar] [CrossRef]
- Goodsell, D.S. Computational docking of biomolecular complexes with Auto-Dock. In Protein-Protein Interactions A Molecular Cloning Manual Second; Golemis, E.A., Adams, P.D., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2005. [Google Scholar]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for ligand-receptor docking. In Current Protocols in Bioinformatics; Wiley, J., Sons, I., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ramos, R.M.; Perez, J.M.; Baptista, L.A.; De Amorim, H.L. Interaction of wild type, G68R and L125M isoforms of the arylamine-N-acetyltransferase from Mycobaerium tuberculosis with isoniazid: A computational study on a new possible mechanism of resistance. J. Mol. Model. 2012, 18, 4013–4024. [Google Scholar] [CrossRef]











| 1H NMR | 13C NMR | ||
|---|---|---|---|
| Troxerutin | δ (ppm) | Troxerutin | δ (ppm) |
| 5-OH | 12.5 (s) | 4-C | 177.9 |
| 2′-Ar | 7.8 (s) | 7-Ar | 165.1 |
| 6′-Ar | 7.7–7.6 (d) | 9-C | 161.3 |
| 5′-Ar | 7.1 (d) | 5-Ar | 157.0 |
| 8-Ar | 6.7 (s) | 2-C | 156.9 |
| 6-Ar | 6.3 (s) | 4′-Ar | 151.3 |
| CH2CH2OH | 5.4–5.3 (m) | 3′-Ar | 148.0 |
| CH2CH2OH | 4.1–4.0 (m) | 3-C | 134.1 |
| CH2CH2OH | 3.5–3.2 (m) | 1′-Ar | 123.0 |
| 6‴-CH3 | 0.9 (m) | 6′-Ar | 122.8 |
| 5′-Ar | 114.8 | ||
| 2′-Ar | 113.2 | ||
| 10-Ar | 105.5 | ||
| 1″-C | 101.7 | ||
| 1‴-C | 101.4 | ||
| 6-Ar | 98.8 | ||
| 3″-C | 76.8 | ||
| 5″-C | 74.6 | ||
| 4‴-C | 72.2 | ||
| 3‴-C | 71.0 | ||
| OCH2CH2OH | 70.8 | ||
| 5‴-C | 68.7 | ||
| CH2OH | 59.9 | ||
| CH2OH | 59.7 | ||
| 6‴-C | 18.2 | ||
| Groups | Scores |
|---|---|
| Saline | 0 (0–1) |
| 5-FU | 3 (2–3) a |
| TRX-50 | 2 (1–3) |
| TRX-100 | 1 (1–2) b |
| TRX-150 | 3 (1–3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Miranda, J.A.L.; Martins, C.d.S.; Fideles, L.d.S.; Barbosa, M.L.L.; Barreto, J.E.F.; Pimenta, H.B.; Freitas, F.O.R.; Pimentel, P.V.d.S.; Teixeira, C.S.; Scafuri, A.G.; et al. Troxerutin Prevents 5-Fluorouracil Induced Morphological Changes in the Intestinal Mucosa: Role of Cyclooxygenase-2 Pathway. Pharmaceuticals 2020, 13, 10. https://doi.org/10.3390/ph13010010
de Miranda JAL, Martins CdS, Fideles LdS, Barbosa MLL, Barreto JEF, Pimenta HB, Freitas FOR, Pimentel PVdS, Teixeira CS, Scafuri AG, et al. Troxerutin Prevents 5-Fluorouracil Induced Morphological Changes in the Intestinal Mucosa: Role of Cyclooxygenase-2 Pathway. Pharmaceuticals. 2020; 13(1):10. https://doi.org/10.3390/ph13010010
Chicago/Turabian Stylede Miranda, João Antônio Leal, Conceição da Silva Martins, Lázaro de Sousa Fideles, Maria Lucianny Lima Barbosa, João Erivan Façanha Barreto, Helder Bindá Pimenta, Francisco Orlando Rafael Freitas, Paulo Vitor de Souza Pimentel, Claudio Silva Teixeira, Ariel Gustavo Scafuri, and et al. 2020. "Troxerutin Prevents 5-Fluorouracil Induced Morphological Changes in the Intestinal Mucosa: Role of Cyclooxygenase-2 Pathway" Pharmaceuticals 13, no. 1: 10. https://doi.org/10.3390/ph13010010
APA Stylede Miranda, J. A. L., Martins, C. d. S., Fideles, L. d. S., Barbosa, M. L. L., Barreto, J. E. F., Pimenta, H. B., Freitas, F. O. R., Pimentel, P. V. d. S., Teixeira, C. S., Scafuri, A. G., dos Santos Luciano, M. C., Araújo, J. L., Rocha, J. A., Vieira, I. G. P., Ricardo, N. M. P. S., da Silva Campelo, M., Ribeiro, M. E. N. P., de Castro Brito, G. A., & Cerqueira, G. S. (2020). Troxerutin Prevents 5-Fluorouracil Induced Morphological Changes in the Intestinal Mucosa: Role of Cyclooxygenase-2 Pathway. Pharmaceuticals, 13(1), 10. https://doi.org/10.3390/ph13010010