Determination of Very Low Concentration of Bisphenol A in Toys and Baby Pacifiers Using Dispersive Liquid–Liquid Microextraction by In Situ Ionic Liquid Formation and High-Performance Liquid Chromatography
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the DLLME Conditions
2.1.1. Optimization of Aqueous Phase and IL Precursors Volume
2.1.2. Optimization of pH Conditions
2.1.3. Optimization of Temperature Conditions and Incubation Time
2.1.4. Optimization of Centrifugation Time
2.2. Analytical Figures of Merit of the Proposed Method
2.3. Application of the Procedure to Toys and Baby Pacifier Samples
3. Materials and Methods
3.1. Chemicals and Materials
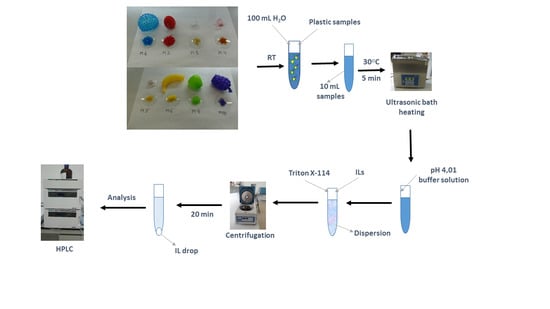
3.2. General Procedure
3.3. Procedure Applied to Plastic Toys and Baby Pacifiers
3.4. Ionic Liquid as Acceptor Phase
3.5. Non-Stick Agent Selection
3.6. Instrumentation and Analytical Conditions
3.7. Microextraction Kinetic Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, D.D.; Gu, Y.Y.; Lan, H.Z.; Sun, Y.Y.; Gao, H.J. Functional graphene-gold nano-composite fabricated electrochemical biosensor for direct and rapid detection of bisphenol A. Anal. Chim. Acta 2015, 853, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Hauser, R. Bisphenol A and children’s health. Curr. Opin. Pediatr. 2011, 23, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenscheing, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Eurpean Union. Commision Regulation (EU) No 2017/898 of 24 May 2017 on Specific Limit Values for Certain Chemical Products Used in Toys. 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017L0898&from=ES (accessed on 3 October 2020).
- Eurpean Comissión. Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food (OJ L 12, 15.1.2011, p.1). 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R0010&from=EN (accessed on 3 October 2020).
- Zhu, S.Q.; Wang, L.J.; Su, A.; Zhang, H.X. Dispersive liquid-liquid microextraction of phenolic compounds from vegetable oils using a magnetic ionic liquid. J. Sep. Sci. 2017, 40, 3130–3137. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Su, P.; Zhang, Y.; Wang, R.Y.; Yang, Y. In-situ ionic liquid-based microwave-assisted dispersive liquid-liquid microextraction of triazine herbicides. Microchim. Acta 2012, 178, 341–347. [Google Scholar] [CrossRef]
- Yao, C.; Anderson, J.L. Dispersive liquid-liquid microextraction using an in situ metathesis reaction to form an ionic liquid extraction phase for the preconcentration of aromatic compounds from water. Anal. Bioanal. Chem. 2009, 395, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, I.; Vicente-Martinez, Y.; Hernandez-Cordoba, M. Determination of lead and cadmium using an ionic liquid and dispersive liquid-liquid microextraction followed by electrothermal atomic absorption spectrometry. Talanta 2013, 110, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, I.; Vicente-Martinez, Y.; Hernandez-Cordoba, M. Determination of very low amounts of chromium(III) and (VI) using dispersive liquid-liquid microextraction by in situ formation of an ionic liquid followed by electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 2012, 27, 874–880. [Google Scholar] [CrossRef]
- Cacho, J.I.; Campillo, N.; Vinas, P.; Hernandez-Cordoba, M. In situ ionic liquid dispersive liquid-liquid microextraction and direct microvial insert thermal desorption for gas chromatographic determination of bisphenol compounds. Anal. Bioanal. Chem. 2016, 408, 243–249. [Google Scholar] [CrossRef]
- Cacho, J.I.; Campillo, N.; Vinas, P.; Hernandez-Cordoba, M. In situ ionic liquid dispersive liquid-liquid microextraction coupled to gas chromatography-mass spectrometry for the determination of organophosphorus pesticides. J. Chromatogr. A 2018, 1559, 95–101. [Google Scholar] [CrossRef]
- Ye, C.L.; Liu, C.; Wang, S.; Wang, Z.K. Investigation of 1-Dodecylimidazolium Modified Filter Papers as a Thin-Film Microextraction Phase for the Preconcentration of Bisphenol A from Plant Oil Samples. Anal. Sci. 2017, 33, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.; Li, T.H.; Twu, P.; Pitner, W.R.; Anderson, J.L. Selective extraction of emerging contaminants from water samples by dispersive liquid-liquid microextraction using functionalized ionic liquids. J. Chromatogr. A 2011, 1218, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Darias, J.; Pino, V.; Ayala, J.H.; Afonso, A.M. In-situ ionic liquid-dispersive liquid-liquid microextraction method to determine endocrine disrupting phenols in seawaters and industrial effluents. Microchim. Acta 2011, 174, 213–222. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Gao, J.; Zhang, H.; Zheng, L.; Chen, J. Determination of bisphenol A from toys and food contact materials by derivatization and gas chromatography-mass spectrometry. Chin. J. Chromatogr. 2012, 30, 1017–1020. [Google Scholar] [CrossRef]
- Fouladgar, M. Nanostructured Sensor for Simultaneous Determination of Trace Amounts of Bisphenol A and Vitamin B-6 in Food Samples. Food Anal. Meth. 2017, 10, 1507–1514. [Google Scholar] [CrossRef]
- Faraji, M.; Noorani, M.; Sahneh, B.N. Quick, Easy, Cheap, Effective, Rugged, and Safe Method Followed by Ionic Liquid-Dispersive Liquid-Liquid Microextraction for the Determination of Trace Amount of Bisphenol A in Canned Foods. Food Anal. Meth. 2017, 10, 764–772. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Liu, S.H.; Xie, Q.L. Rapid determination of phthalate esters in alcoholic beverages by conventional ionic liquid dispersive liquid-liquid microextraction coupled with high performance liquid chromatography. Talanta 2014, 119, 291–298. [Google Scholar] [CrossRef]
- Hu, Y.J.; Liu, Z.M.; Zhan, H.J.; Hu, L.Q.; Cui, L.; Wang, K. A novel electrochemiluminescence sensor for bisphenol A determination based on graphene-palladium nanoparticles/polyvinyl alcohol hybrids. Anal. Methods 2017, 9, 3870–3875. [Google Scholar] [CrossRef]
- Ben Messaoud, N.; Ghica, M.E.; Dridi, C.; Ben Ali, M.; Brett, C.M.A. A novel amperometric enzyme inhibition biosensor based on xanthine oxidase immobilised onto glassy carbon electrodes for bisphenol A determination. Talanta 2018, 184, 388–393. [Google Scholar] [CrossRef]
- Negev, M.; Berman, T.; Reicher, S.; Sadeh, M.; Ardi, R.; Shammai, Y. Concentrations of trace metals, phthalates, bisphenol A and flame-retardants in toys and other children’s products in Israel. Chemosphere 2018, 192, 217–224. [Google Scholar] [CrossRef]
- Wooten, K.J.; Smith, P.N. Canine toys and training devices as sources of exposure to phthalates and bisphenol A: Quantitation of chemicals in leachate and in vitro screening for endocrine activity. Chemosphere 2013, 93, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Eurpean Regulations. EN 71-10:2005. Safety of Toys. Organic Chemical Compounds. 2005. Available online: https://ilnas.services-publics.lu/ecnor/downloadPreview.action?documentReference=45158 (accessed on 4 October 2020).
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Almeida, S.; Raposo, A.; Almeida-Gonzalez, M.; Carrascosa, C. Bisphenol A: Food Exposure and Impact on Human Health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef]
- Bolognesi, C.; Castle, L.; Cravedi, J.P.; Engel, K.H.; Fowler, P.; Franz, R.; Grob, K.; Gurtler, R.; Husoy, T.; Mennes, W.; et al. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA J. 2015, 13, 3978. [Google Scholar]
- Andaluri, G.; Manickavachagam, M.; Suri, R. Plastic toys as a source of exposure to bisphenol-A and phthalates at childcare facilities. Environ. Monit. Assess. 2018, 190, 65. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, E.; Santoni, E.; Vittori, S.; Font, G.; Manes, J.; Sagratini, G. Simultaneous determination of bisphenol A, octylphenol, and nonylphenol by pressurised liquid extraction and liquid chromatography-tandem mass spectrometry in powdered milk and infant formulas. Food Chem. 2011, 126, 360–367. [Google Scholar] [CrossRef]
- Dorival-Garcia, N.; Zafra-Gomez, A.; Navalon, A.; Vilchez, J.L. Improved sample treatment for the determination of bisphenol A and its chlorinated derivatives in sewage sludge samples by pressurized liquid extraction and liquid chromatography-tandem mass spectrometry. Talanta 2012, 101, 1–10. [Google Scholar] [CrossRef]
- Tu, X.J.; Wu, S.Y.; Liu, W.Y.; Gao, Z.S.; Huang, S.K.; Chen, W.B. Sugaring-Out Assisted Liquid-Liquid Extraction Combined with High-Performance Liquid Chromatography-Fluorescence Detection for the Determination of Bisphenol A and Bisphenol B in Royal Jelly. Food Anal. Meth. 2019, 12, 705–711. [Google Scholar] [CrossRef]
- Notardonato, I.; Passarella, S.; Ianiri, G.; Di Fiore, C.; Russo, M.V.; Avino, P. Analytical Scheme for Simultaneous Determination of Phthalates and Bisphenol A in Honey Samples Based on Dispersive Liquid-Liquid Microextraction Followed by GC-IT/MS. Effect of the Thermal Stress on PAE/BP-A Levels. Methods Protoc. 2020, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Amini, R.; Khandaghi, J.; Mogaddam, M.R.A. Combination of Vortex-Assisted Liquid-Liquid Extraction and Air-Assisted Liquid-Liquid Microextraction for the Extraction of Bisphenol A and Bisphenol B in Canned Doogh Samples. Food Anal. Meth. 2018, 11, 3267–3275. [Google Scholar] [CrossRef]
- Mo, R.H.; Liu, H.X.; Lai, R.T.; Deng, G.F.; Zhang, Z.Q.; Pei, Z.Z.; Li, H.B.; Xia, E.Q. Ultrasound-Assisted Upper Liquid Microextraction Coupled to Molecular Fluorescence for Detection of Bisphenol A in Commercial Beverages. Food Anal. Meth. 2017, 10, 1575–1581. [Google Scholar] [CrossRef]
- Nascimento, C.F.; Rocha, F.R.P. Spectrofluorimetric determination of bisphenol A in tap waters by exploiting liquid-liquid microextraction in a sequential injection system. Microchem. J. 2018, 137, 429–434. [Google Scholar] [CrossRef]
- Freire, M.G.; Santos, L.; Fernandes, A.M.; Coutinho, J.A.P.; Marrucho, I.M. An overview of the mutual solubilities of water-imidazolium-based ionic liquids systems. Fluid Phase Equilibria 2007, 261, 449–454. [Google Scholar] [CrossRef]
- Lopez-Garcia, I.; Vicente-Martinez, Y.; Hernandez-Cordoba, M. Cloud point extraction assisted by silver nanoparticles for the determination of traces of cadmium using electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 2015, 30, 375–380. [Google Scholar] [CrossRef]
- Vicente-Martínez, Y.; Caravaca, M.; Soto-Meca, A.; De Francisco-Ortiz, O.; Gimeno, F. Graphene oxide and graphene oxide functionalized with silver nanoparticles as adsorbents of phosphates in waters. A comparative study. Sci. Total Environ. 2020, 709, 136111. [Google Scholar] [CrossRef]




| Aqueous Phase Volume (mL) | IL Precursors Volume (μL) | IL Volume after Centrifugation (μL) | EF |
|---|---|---|---|
| 5 | 100 | 48 | 104 |
| 10 | 100 | 33 | 303 |
| 15 * | 100 | 18 | 833 |
| 20 * | 100 | 10 | 2000 |
| Sample of Known Concentration | Concentration (µg L−1) | Content Found a (µg L−1) |
|---|---|---|
| blank | 0.0 | 0.01 ± 0.00 |
| 1 | 0.30 | 0.303 ± 0.002 |
| 2 | 0.40 | 0.401 ± 0.001 |
| 3 | 0.50 | 0.500 ± 0.003 |
| Sample | Content Found a (µg L−1) | Recovery % |
|---|---|---|
| 1—Blue ball | 0.30 ± 0.02 | 100 |
| 2—Red ball | ND | 101 |
| 3—White teat | 0.23 ± 0.01 | 97 |
| 4—Pink teat | ND | - |
| 5—Red teat | ND | - |
| 6—Yellow banana | 0.25 ± 0.01 | 102 |
| 7—Green apple | 0.22 ± 0.04 | 101 |
| 8—Violet grape | 0.30 ± 0.03 | 99 |
| 9—Blue ball | 0.28 ± 0.02 | 98 |
| 10—Yellow horse | 0.21 ± 0.02 | 101 |
| 11—Orange giraffe | 0.25 ± 0.04 | 98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente-Martínez, Y.; Caravaca, M.; Soto-Meca, A. Determination of Very Low Concentration of Bisphenol A in Toys and Baby Pacifiers Using Dispersive Liquid–Liquid Microextraction by In Situ Ionic Liquid Formation and High-Performance Liquid Chromatography. Pharmaceuticals 2020, 13, 301. https://doi.org/10.3390/ph13100301
Vicente-Martínez Y, Caravaca M, Soto-Meca A. Determination of Very Low Concentration of Bisphenol A in Toys and Baby Pacifiers Using Dispersive Liquid–Liquid Microextraction by In Situ Ionic Liquid Formation and High-Performance Liquid Chromatography. Pharmaceuticals. 2020; 13(10):301. https://doi.org/10.3390/ph13100301
Chicago/Turabian StyleVicente-Martínez, Yesica, Manuel Caravaca, and Antonio Soto-Meca. 2020. "Determination of Very Low Concentration of Bisphenol A in Toys and Baby Pacifiers Using Dispersive Liquid–Liquid Microextraction by In Situ Ionic Liquid Formation and High-Performance Liquid Chromatography" Pharmaceuticals 13, no. 10: 301. https://doi.org/10.3390/ph13100301
APA StyleVicente-Martínez, Y., Caravaca, M., & Soto-Meca, A. (2020). Determination of Very Low Concentration of Bisphenol A in Toys and Baby Pacifiers Using Dispersive Liquid–Liquid Microextraction by In Situ Ionic Liquid Formation and High-Performance Liquid Chromatography. Pharmaceuticals, 13(10), 301. https://doi.org/10.3390/ph13100301