Gel-Based Nanocarrier for Intravesical Chemotherapy Delivery: In Vitro and In Vivo Study
Abstract
:1. Introduction
2. Results and Discussion
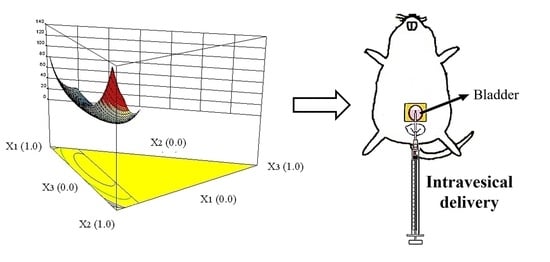
2.1. Characteristics of Formulations
2.2. In Vitro Permeation and Accumulation Study
2.3. In Vivo Intravesical Instillation of Gemcitabine
3. Materials and Methods
3.1. Materials and Animals
3.2. Drug-Loaded Nanocarrier Preparations
3.3. Characterize of Formulations
3.4. In Vitro Permeation
3.5. In Vivo Intravesical Administration of Gemcitabine-Loaded Formulation
3.6. Chromatographic Condition
3.7. Penetration Depth Measurement
3.8. Data Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Babjuk, M.; Bohle, A.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Hernandez, V.; Kaasinen, E.; Palou, J.; Roupret, M.; et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Neoh, K.G.; Kang, E.T.; Mahendran, R.; Chiong, E. Mucoadhesive polyacrylamide nanogel as a potential hydrophobic drug carrier for intravesical bladder cancer therapy. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2015, 72, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Neutsch, L.; Wirth, E.M.; Spijker, S.; Pichl, C.; Kahlig, H.; Gabor, F.; Wirth, M. Synergistic targeting/prodrug strategies for intravesical drug delivery--lectin-modified PLGA microparticles enhance cytotoxicity of stearoyl gemcitabine by contact-dependent transfer. J. Control. Release Off. J. Control. Release Soc. 2013, 169, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Falke, J.; Hulsbergen-van de Kaa, C.A.; Maj, R.; Oosterwijk, E.; Witjes, J.A. Pharmacokinetics and pharmacodynamics of intravesical and intravenous TMX-101 and TMX-202 in a F344 rat model. Urol. Oncol. 2018, 36, 242 e1–242 e7. [Google Scholar] [CrossRef]
- Lopedota, A.; Cutrignelli, A.; Laquintana, V.; Denora, N.; Iacobazzi, R.M.; Perrone, M.; Fanizza, E.; Mastrodonato, M.; Mentino, D.; Lopalco, A.; et al. Spray Dried Chitosan Microparticles for Intravesical Delivery of Celecoxib: Preparation and Characterization. Pharm. Res. 2016, 33, 2195–2208. [Google Scholar] [CrossRef]
- Chou, R.; Selph, S.; Buckley, D.I.; Fu, R.; Griffin, J.C.; Grusing, S.; Gore, J.L. Intravesical Therapy for the Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. J. Urol. 2017, 197, 1189–1199. [Google Scholar] [CrossRef]
- Bergman, A.M.; Pinedo, H.M.; Peters, G.J. Determinants of resistance to 2′,2′-difluorodeoxycytidine (gemcitabine). Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2002, 5, 19–33. [Google Scholar] [CrossRef]
- Liu, P.; Rong, X.; Laru, J.; van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Laaksonen, T.; Peltonen, L. Nanosuspensions of poorly soluble drugs: Preparation and development by wet milling. Int. J. Pharm. 2011, 411, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Tuan-Mahmood, T.M.; McCrudden, M.T.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanun, M. Phase behavior, transport, diffusion and structural parameters of nonionic surfactants microemulsions. J. Mol. Liq. 2008, 139, 1–3. [Google Scholar] [CrossRef]
- Duangjit, S.; Chairat, W.; Opanasopit, P.; Rojanarata, T.; Panomsuk, S.; Ngawhirunpat, T. Development, Characterization and Skin Interaction of Capsaicin-Loaded Microemulsion-Based Nonionic Surfactant. Biol. Pharm. Bull. 2016, 39, 601–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdal, M.S.; Ozhan, G.; Mat, M.C.; Ozsoy, Y.; Gungor, S. Colloidal nanocarriers for the enhanced cutaneous delivery of naftifine: Characterization studies and in vitro and in vivo evaluations. Int. J. Nanomed. 2016, 11, 1027–1037. [Google Scholar] [CrossRef] [Green Version]
- Naeem, M.; Ur Rahman, N.; Tavares, G.D.; Barbosa, S.F.; Chacra, N.B.; Lobenberg, R.; Sarfraz, M.K. Physicochemical, in vitro and in vivo evaluation of flurbiprofen microemulsion. An. Da Acad. Bras. De Cienc. 2015, 87, 1823–1831. [Google Scholar] [CrossRef] [Green Version]
- Todosijevic, M.N.; Savic, M.M.; Batinic, B.B.; Markovic, B.D.; Gasperlin, M.; Randelovic, D.V.; Lukic, M.Z.; Savic, S.D. Biocompatible microemulsions of a model NSAID for skin delivery: A decisive role of surfactants in skin penetration/irritation profiles and pharmacokinetic performance. Int. J. Pharm. 2015, 496, 931–941. [Google Scholar] [CrossRef] [Green Version]
- Mahrhauser, D.S.; Kahlig, H.; Partyka-Jankowska, E.; Peterlik, H.; Binder, L.; Kwizda, K.; Valenta, C. Investigation of microemulsion microstructure and its impact on skin delivery of flufenamic acid. Int. J. Pharm. 2015, 490, 292–297. [Google Scholar] [CrossRef]
- Otto, A.; Wiechers, J.W.; Kelly, C.L.; Hadgraft, J.; du Plessis, J. Effect of penetration modifiers on the dermal and transdermal delivery of drugs and cosmetic active ingredients. Skin Pharmacol. Physiol. 2008, 21, 326–334. [Google Scholar] [CrossRef]
- Okabe, K.; Kimura, H.; Okabe, J.; Kato, A.; Shimizu, H.; Ueda, T.; Shimada, S.; Ogura, Y. Effect of benzalkonium chloride on transscleral drug delivery. Investig. Ophthalmol. Vis. Sci. 2005, 46, 703–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Bijl, P.; Van Eyk, A.D.; Gareis, A.A.; Thompson, I.O. Enhancement of transmucosal permeation of cyclosporine by benzalkonium chloride. Adv. Exp. Med. Biol. 2003, 528, 567–570. [Google Scholar]
- Tsai, M.J.; Lu, I.J.; Fu, Y.S.; Fang, Y.P.; Huang, Y.B.; Wu, P.C. Nanocarriers enhance the transdermal bioavailability of resveratrol: In-vitro and in-vivo study. Colloids Surf. B Biointerfaces 2016, 148, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sinha, V.R. Preparation and optimization of voriconazole microemulsion for ocular delivery. Colloids Surf. B Biointerfaces 2014, 117, 82–88. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, G.M. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: Effects of cosurfactants. Int. J. Pharm. 2008, 355, 285–292. [Google Scholar] [CrossRef]
- Koenig, F.; Knittel, J.; Schnieder, L.; George, M.; Lein, M.; Schnorr, D. Confocal laser scanning microscopy of urinary bladder after intravesical instillation of a fluorescent dye. Urology 2003, 62, 158–161. [Google Scholar] [CrossRef]
- Zou, Y.; Celli, A.; Zhu, H.; Elmahdy, A.; Cao, Y.; Hui, X.; Maibach, H. Confocal laser scanning microscopy to estimate nanoparticles’ human skin penetration in vitro. Int. J. Nanomed. 2017, 12, 8035–8041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.H.; Hsieh, Y.H.; Huang, Y.B.; Chang, J.S.; Huang, C.T.; Wu, P.C. Microemulsions for intravesical delivery of gemcitabine. Chem. Pharm. Bull. 2010, 58, 1461–1465. [Google Scholar] [CrossRef] [Green Version]



| X1 Code | X2 Code | X3 Code | Size nm | PDI | Viscosity (cps) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F01 | 0.50 | 0.43 | 0.07 | 57.4 | ± | 0.1 | 0.05 | ± | 0.00 | 481.0 | ± | 6.5 |
| F02 | 0.50 | 0.43 | 0.07 | 55.3 | ± | 5.3 | 0.19 | ± | 0.01 | 483.0 | ± | 11.0 |
| F03 | 0.88 | 0.00 | 0.12 | 163.5 | ± | 2.4 | 0.35 | ± | 0.02 | 407.2 | ± | 15.7 |
| F04 | 0.95 | 0.04 | 0.01 | 10.5 | ± | 1.4 | 0.20 | ± | 0.03 | 661.1 | ± | 13.8 |
| F05 | 0.40 | 0.60 | 0.00 | 76.2 | ± | 3.1 | 0.20 | ± | 0.01 | 464.7 | ± | 5.3 |
| F06 | 0.24 | 0.76 | 0.00 | 141.9 | ± | 14.1 | 0.11 | ± | 0.01 | 326.6 | ± | 14.9 |
| F07 | 0.23 | 0.63 | 0.14 | 168.0 | ± | 46.1 | 0.14 | ± | 0.01 | 330.9 | ± | 20.3 |
| F08 | 0.00 | 0.92 | 0.08 | 165.9 | ± | 40.5 | 0.13 | ± | 0.02 | 274.1 | ± | 8.8 |
| Variables | Code Level Low | Code Level High |
|---|---|---|
| X1: 1,5-Pentanediol 10~20% | 0.0 | 0.6 |
| X2: Carbitol 10~20% | 0.0 | 1.0 |
| X3: Benzalkonium chloride 1.5~3% | 0.0 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-Y.; Tsai, M.-J.; Lin, I.-L.; Chang, L.-C.; Wu, P.-C. Gel-Based Nanocarrier for Intravesical Chemotherapy Delivery: In Vitro and In Vivo Study. Pharmaceuticals 2020, 13, 329. https://doi.org/10.3390/ph13110329
Chen T-Y, Tsai M-J, Lin I-L, Chang L-C, Wu P-C. Gel-Based Nanocarrier for Intravesical Chemotherapy Delivery: In Vitro and In Vivo Study. Pharmaceuticals. 2020; 13(11):329. https://doi.org/10.3390/ph13110329
Chicago/Turabian StyleChen, Ting-Yu, Ming-Jun Tsai, I-Ling Lin, Li-Ching Chang, and Pao-Chu Wu. 2020. "Gel-Based Nanocarrier for Intravesical Chemotherapy Delivery: In Vitro and In Vivo Study" Pharmaceuticals 13, no. 11: 329. https://doi.org/10.3390/ph13110329
APA StyleChen, T.-Y., Tsai, M.-J., Lin, I.-L., Chang, L.-C., & Wu, P.-C. (2020). Gel-Based Nanocarrier for Intravesical Chemotherapy Delivery: In Vitro and In Vivo Study. Pharmaceuticals, 13(11), 329. https://doi.org/10.3390/ph13110329