Brain-Selective Estrogen Therapy Prevents Androgen Deprivation-Associated Hot Flushes in a Rat Model
Abstract
:1. Introduction
2. Results
2.1. E2 Concentration Increased in the Hypothalamus without Increasing Serum Estrogen after Oral Administration of DHED
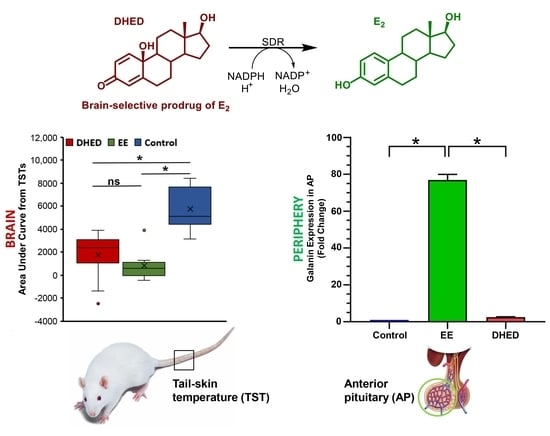
2.2. DHED Treatment Blunted Increase of TST in ORDX Male Rats in a Pharmacological Model of Hot Flushes
2.3. DHED Treatment Induced Progesterone Receptor (PR) Expression in the Preoptic Area (POA) of the Hypothalamus
2.4. DHED Treatment Did Not Impact Galanin Gene Expression in the Anterior Pituitary (AP)
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Measurement of E2 in the Hypothalamus and Serum after Oral Administration of DHED
4.4. Direct TST Measurements in ORDX Morphine-Dependent Rats
4.5. In Situ Hybridization Histochemistry (ISHH)
4.6. Quantitative RT-PCR (qRT-PCR) for Galanin Expression in the Anterior Pituitary (AP)
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sturdee, D.W.; Hunter, M.S.; Maki, P.M.; Gupta, P.; Sassarini, J.; Stevenson, J.C.; Lumsden, M.A. The menopausal hot flush: A review. Climacteric 2017, 20, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Epperson, C.N.; Mathews, S.B. Menopausal symptoms and their management. Endocrinol. Metab. Clin. N. Am. 2015, 44, 497–515. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, E.; Schellhammer, P.; Wassersug, R.J. Role of estrogen in normal male function: Clinical implications for patients with prostate cancer on androgen deprivation therapy. J. Urol. 2010, 185, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Spetz, A.-C.E.; Fredriksson, M.; Hammar, M.L. Hot flushes in a male population aged 55, 65, and 75 years, living in the community of Linköping, Sweden. Menopause 2003, 10, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.P.; Lee, H.; Webb, M.L.; Joffe, H.; Finkelstein, J.S. Effects of testosterone and estradiol deficiency on vasomotor symptoms in hypogonadal men. J. Clin. Endocrinol. Metab. 2016, 101, 3479–3486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagouri, F.; Sergentanis, T.N.; Azim, H.A., Jr.; Chrysikos, D.; Dimopoulos, M.A.; Psaltopoulou, T. Aromatase inhibitors in male breast cancer: A pooled analysis. Breast Cancer Res. Treat. 2015, 151, 141–147. [Google Scholar] [CrossRef] [PubMed]
- De Ronde, W.; de Jong, F.H. Aromatase inhibitors in men: Effects and therapeutic options. Reprod. Biol. Endocrinol. 2011, 9, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, E.; Rubin, G.; Clyne, C.; Robertson, K.; O’Donnell, L.; Jones, M.; Davis, S. The role of local estrogen biosynthesis in males and females. Trends Endocrinol. Metab. 2000, 11, 184–188. [Google Scholar] [CrossRef]
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A. Estrogens in male physiology. Physiol. Rev. 2013, 97, 995–1043. [Google Scholar] [CrossRef] [PubMed]
- Diotel, N.; Charlier, T.D.; Lefebvre d’Hellencourt, C.; Couret, D.; Trudeau, V.L.; Nicolau, J.C.; Meilhac, O.; Kah, O.; Pellegrini, E. Steroid transport, local synthesis, and signaling within the brain: Roles in neurogenesis, neuroprotection, and sexual behaviors. Front. Neurosci. 2018, 12, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S. Estrogens effects on the brain: Multiple sites and molecular mechanisms. J. Appl. Physiol. 2001, 91, 2785–2801. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.; Grossmann, M. Estradiol as a male hormone. Eur. J. Endocrinol. 2019, 181, R23–R43. [Google Scholar] [CrossRef] [PubMed]
- Gerber, G.S.; Zagaja, G.P.; Ray, P.S.; Rukstalis, D.B. Transdermal estrogen in the treatment of hot flushes in men with prostate cancer. Urology 2000, 55, 97–101. [Google Scholar] [CrossRef]
- Sciarra, A.; Gentile, V.; Cattarino, S.; Gentilucci, A.; Alfarone, A.; D’Eramo, G.; Salciccia, S. Oral ethinylestradiol in castration-resistant prostate cancer: A 10-year experience. Int. J. Urol. 2015, 22, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Eastham, J.; Shore, N. Androgen deprivation therapy and estrogen deficiency induced adverse effects in the treatment of prostate cancer. Prostate Cancer Prost. Dis. 2009, 12, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokai, L.; Nguyen, V.; Szarka, S.; Garg, P.; Sabnis, G.; Bimonte-Nelson, H.A.; McLaughlin, K.J.; Talboom, J.S.; Conrad, C.D.; Shughrue, P.J.; et al. The prodrug DHED selectively delivers 17β-estradiol to the brain for treating estrogen-responsive disorders. Sci. Transl. Med. 2015, 7, 297ra113. [Google Scholar] [CrossRef] [Green Version]
- Merchenthaler, I.; Lane, M.; Sabnis, G.; Brodie, A.; Nguyen, V.; Prokai, L.; Prokai-Tatrai, K. Treatment with an orally bioavailable prodrug of 17β-estradiol alleviates hot flushes without hormonal effects in the periphery. Sci. Rep. 2016, 6, 30721. [Google Scholar] [CrossRef] [Green Version]
- Prokai-Tatrai, K.; Prokai, L. A novel prodrug approach for central nervous system-selective estrogen therapy. Molecules 2019, 24, 4197. [Google Scholar] [CrossRef] [Green Version]
- Prokai-Tatrai, K.; Nguyen, V.; De La Cruz, D.L.; Guerra, R.; Rahlouni, F.; Prokai, L. Retina-targeted delivery of 17β-estradiol by the topically applied DHED prodrug. Pharmaceutics 2020, 12, 456. [Google Scholar] [CrossRef] [PubMed]
- Key Statistics for Prostate Cancer. American Cancer Society. Available online: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html (accessed on 18 April 2020).
- Merchenthaler, I.; Stennett, C.A.; Haughey, B.; Puche, A.; Urbanski, H.F. Establishment of a non-human primate model for menopausal hot flushes. EC Gynaecol. 2019, 9, 1–7. [Google Scholar]
- Dierschke, D.J. Temperature changes suggestive of hot flushes in rhesus monkeys: Preliminary observations. J. Med. Primat. 1985, 14, 271–280. [Google Scholar]
- Jelinek, J.; Kappen, A.; Schönbaum, E.; Lomax, P. A primate model of human postmenopausal hot flushes. J. Clin. Endocrinol. Metab. 1984, 59, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Albertson, A.J.; Skinner, D.C. A novel animal model to study hot flashes: No effect of GnRH. Menopause 2009, 16, 1030–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merchenthaler, I.; Funkhouser, J.M.; Carver, J.M.; Lundeen, S.G.; Ghosh, K.; Winneker, R.C. The effect of estrogens and antiestrogens in a rat model for hot flush. Maturitas 1998, 30, 307–316. [Google Scholar] [CrossRef]
- Williams, H.; Dacks, P.A.; Rance, N.E. An improved method for recording tail skin temperature in the rat reveals changes during the estrous cycle and effects of ovarian steroids. Endocrinology 2010, 151, 5389–5394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merchenthaler, I. The effect of estrogens and antiestrogens in rat models of hot flush. Drug Dev. Res. 2006, 66, 182–188. [Google Scholar] [CrossRef]
- Szarka, S.; Nguyen, V.; Prokai, L.; Prokai-Tatrai, K. Separation of dansylated 17β-estradiol, 17α-estradiol, and estrone on a single HPLC column for simultaneous quantitation by LC-MS/MS. Anal. Bioanal. Chem. 2013, 405, 3399–3406. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.R. Physiology of hot flashes. Am. J. Hum. Biol. 2001, 13, 453–464. [Google Scholar] [CrossRef]
- Shughrue, P.J.; Lane, M.V.; Merchenthaler, I. Regulation of progesterone receptor messenger ribonucleic acid in the rat medial preoptic nucleus by estrogenic and antiestrogenic compounds: An in situ hybridization study. Endocrinology 1997, 138, 5476–5484. [Google Scholar] [CrossRef]
- Kaplan, L.M.; Gabriel, S.M.; Koenig, J.I.; Sunday, M.E.; Spindel, E.R.; Martin, J.B.; Chin, W.W. Galanin is an estrogen-inducible, secretory product of the rat anterior pituitary. Proc. Natl. Acad. Sci. USA 1998, 85, 7408–7412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merchenthaler, I.; Lane, M.; Stennett, C.; Zhan, M.; Nguyen, V.; Prokai-Tatrai, K.; Prokai, L. Brain-Selective Estrogen Therapy Prevents Androgen Deprivation-Associated Hot Flushes in a Rat Model. Pharmaceuticals 2020, 13, 119. https://doi.org/10.3390/ph13060119
Merchenthaler I, Lane M, Stennett C, Zhan M, Nguyen V, Prokai-Tatrai K, Prokai L. Brain-Selective Estrogen Therapy Prevents Androgen Deprivation-Associated Hot Flushes in a Rat Model. Pharmaceuticals. 2020; 13(6):119. https://doi.org/10.3390/ph13060119
Chicago/Turabian StyleMerchenthaler, Istvan, Malcolm Lane, Christina Stennett, Min Zhan, Vien Nguyen, Katalin Prokai-Tatrai, and Laszlo Prokai. 2020. "Brain-Selective Estrogen Therapy Prevents Androgen Deprivation-Associated Hot Flushes in a Rat Model" Pharmaceuticals 13, no. 6: 119. https://doi.org/10.3390/ph13060119
APA StyleMerchenthaler, I., Lane, M., Stennett, C., Zhan, M., Nguyen, V., Prokai-Tatrai, K., & Prokai, L. (2020). Brain-Selective Estrogen Therapy Prevents Androgen Deprivation-Associated Hot Flushes in a Rat Model. Pharmaceuticals, 13(6), 119. https://doi.org/10.3390/ph13060119