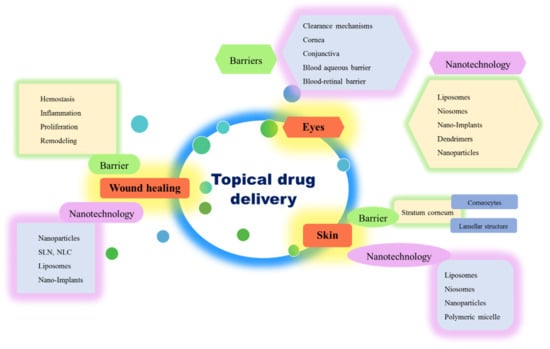
Evolution of Nanotechnology in Delivering Drugs to Eyes, Skin and Wounds via Topical Route
Abstract
:1. Introduction
2. Nanotechnology in Ocular Drug Delivery
2.1. Anatomical Structure of Eye
2.2. Barriers for Topical Drug Delivery to Eyes
2.3. Nanotechnology in Overcoming the Barriers of Topical Delivery to the Eye
2.4. Nanotechnology-Driven Drug Carriers for Topical Delivery to Eyes
2.4.1. Liposomes
- (1)
- Adsorption: Adsorption of liposomes onto the cell membrane is the first step in delivering the drugs from the liposomes. In the presence of cell surface proteins, liposomes become leaky and release their contents near the cell membrane. This results in a high drug concentration in the vicinity of the cell membrane, and promotes the cellular uptake of drugs by passive diffusion [26].
- (2)
- Endocytosis: After adsorption and cellular uptake, the liposome reaches into the endosomes, and is then transported to the lysosomes through endosomes. Later, the enzymes in the lysosomes degrade the lipids and the entrapped drug will be released into the cytoplasm [26].
- (3)
- Fusion: Fusion of the lipid bilayer of liposomes with lipoidal cell membrane by intermixing and lateral diffusion of lipids results in direct delivery of liposomal contents into the cytoplasm [26].
- (4)
- Lipid exchange: Due to the similarity in the lipids present in the liposomal membrane and the phospholipids present in the cell membrane, lipid transfer proteins in the cell membrane recognize liposomes and therefore cause lipid exchange. As a result of this, the liposomal membrane gets destabilized and the drug gets released [26].
2.4.2. Niosomes
2.4.3. Nanoparticles
2.4.4. Polymeric Micelles
2.4.5. Dendrimers
2.4.6. Nano-Implants
3. Topical Drug Delivery to Skin
3.1. Anatomy of Skin
3.2. Barriers for Topical Drug Delivery to Skin
3.3. Conventional Topical Delivery Systems in Treating Skin Diseases
3.4. Nanotechnology-Driven Topical Drug Delivery Systems for Skin
3.5. Types of Nanotechnology-Driven Drug Delivery Systems Available for Delivering Drugs to Skin
3.5.1. Liposomes
3.5.2. Solid Lipid Nanoparticles
3.5.3. Niosomes
3.5.4. Nanoparticles
3.5.5. Polymeric Micelles
4. Wounds and the Barriers for Topical Drug Delivery to Wounds
4.1. The Process of Wound Healing
4.2. Role of Nanotechnology in Wound Healing
4.2.1. Nanoparticles
4.2.2. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
4.2.3. Liposomes
4.2.4. Nano Implants
5. Toxicological Aspects of Topically Applied Nanoformulations
6. Conclusions
7. Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jensen, L.; Petersson, K.; Nielsen, H.M. In vitro penetration properties of solid lipid nanoparticles in intact and barrier-impaired skin. Eur. J. Pharm. Biopharm. 2011, 79, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Sharma, O.P.; Mehta, T. Nanocrystal: A novel approach to overcome skin barriers for improved topical drug delivery. Expert Opin. Drug Deliv. 2018, 15, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.; García, M. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye–Part I–Barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Reimondez-Troitiño, S.; Csaba, N.; Alonso, M.J.; De La Fuente, M. Nanotherapies for the treatment of ocular diseases. Eur. J. Pharm. Biopharm. 2015, 95, 279–293. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2015, 240, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Barry, B.W. Breaching the skin’s barrier to drugs. Nat. Biotechnol. 2004, 22, 165–167. [Google Scholar] [CrossRef]
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Ita, K.B. Prodrugs for transdermal drug delivery-trends and challenges. J. Drug Target. 2016, 24, 671–678. [Google Scholar] [CrossRef]
- Charoenputtakun, P.; Li, S.K.; Ngawhirunpat, T. Iontophoretic delivery of lipophilic and hydrophilic drugs from lipid nanoparticles across human skin. Int. J. Pharm. 2015, 495, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.E.; Mcildowie, M.; Prow, T.W. Magnetophoresis: Skin Penetration Enhancement by a Magnetic Field. In Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2017; pp. 195–206. [Google Scholar]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 2019. [Google Scholar]
- Radhakrishnan, A.; Kuppusamy, G.; Karri, V.V.S.R. Spray bandage strategy in topical drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 113–121. [Google Scholar] [CrossRef]
- Ingle, A.P.; Paralikar, P.; Grupenmacher, A.; Padovani, F.H.; Ferrer, M.T.; Rai, M.K.; Alves, M. Nanotechnological Interventions for Drug Delivery in Eye Diseases. In Nanotechnology Applied To Pharmaceutical Technology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 279–306. [Google Scholar]
- Jiang, S.; Franco, Y.L.; Zhou, Y.; Chen, J. Nanotechnology in retinal drug delivery. Int. J. Ophthalmol. 2018, 11, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Patel, V.; Mehta, T. Nanocrystal for ocular drug delivery: Hope or hype. Drug Deliv. Transl. Res. 2016, 6, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.-Q.; Godley, B.F. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: A possible mechanism for RPE aging and age-related macular degeneration. Exp. Eye Res. 2003, 76, 397–403. [Google Scholar] [CrossRef]
- Mehra, N.K.; Cai, D.; Kuo, L.; Hein, T.; Palakurthi, S. Safety and toxicity of nanomaterials for ocular drug delivery applications. Nanotoxicology 2016, 10, 397–403. [Google Scholar] [CrossRef]
- Liu, S.; Jones, L.; Gu, F. Nanomaterials for Ocular Drug Delivery. Macromol. Biosci. 2012, 12, 608–620. [Google Scholar] [CrossRef]
- Kompella, U.B.; Amrite, A.C.; Ravi, R.P.; Durazo, S.A. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog. Retin. Eye Res. 2013, 36, 172–198. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, C.-H.; Ji, T.; Mehta, M.; Wang, W.; Marino, E.; Chen, J.; Kohane, D.S. Intravenous treatment of choroidal neovascularization by photo-targeted nanoparticles. Nat. Commun. 2019, 10, 804. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sun, D.; Fan, Q.; Ma, Q.; Dong, Z.; Tao, W.; Tao, H.; Liu, Z.; Wang, C. The enhanced permeability and retention effect based nanomedicine at the site of injury. Nano Res. 2020, 13, 564–569. [Google Scholar] [CrossRef]
- Mody, N.; Tekade, R.K.; Mehra, N.K.; Chopdey, P.; Jain, N.K. Dendrimer, Liposomes, Carbon Nanotubes and PLGA Nanoparticles: One Platform Assessment of Drug Delivery Potential. AAPS PharmSciTech 2014, 15, 388–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Nasir, N.A.A.; Razali, N.; Alyautdin, R.; Ismail, N.M. Liposomes in topical ophthalmic drug delivery: An update. Drug Deliv. 2014, 23, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Eloy, J.O.; Petrilli, R.; Trevizan, L.N.F.; Chorilli, M. Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 454–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, L.; Seifert, O.; Vollmer, S.; Kontermann, R.E.; Schlosshauer, B.; Hartmann, H. Immunoliposomes for Targeted Delivery of an Antifibrotic Drug. Mol. Pharm. 2015, 12, 3146–3157. [Google Scholar] [CrossRef]
- Sandeep, D.; AlSawaftah, N.M.; Husseini, G.A. Immunoliposomes: Synthesis, Structure, and Their Potential as Drug Delivery Carriers. Curr. Cancer Ther. Rev. 2020, 16, 1–14. [Google Scholar] [CrossRef]
- Rodríguez, A.; Del, A.; Angeles, M. Non-Viral Delivery Systems in Gene Therapy. In Gene Therapy-Tools and Potential Applications; IntechOpen: London, UK, 2013. [Google Scholar]
- Zuidam, N.J.; Barenholz, Y. Electrostatic and structural properties of complexes involving plasmid DNA and cationic lipids commonly used for gene delivery. Biochim. Biophys. Acta Biomembr. 1998, 1368, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Harada, A.; Sakanaka, K.; Nishida, K.; Nakamura, J.; Sakaeda, T.; Ichikawa, N.; Nakashima, M.; Sasaki, H. In vivo gene transfection via intravitreal injection of cationic liposome/plasmid DNA complexes in rabbits. Int. J. Pharm. 2004, 278, 255–262. [Google Scholar] [CrossRef]
- Liu, H.-A.; Liu, Y.-L.; Ma, Z.-Z.; Wang, J.-C.; Zhang, Q. A Lipid Nanoparticle System Improves siRNA Efficacy in RPE Cells and a Laser-Induced Murine CNV Model. Investig. Opthalmol. Vis. Sci. 2011, 52, 4789–4794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujisawa, T.; Miyai, H.; Hironaka, K.; Tsukamoto, T.; Tahara, K.; Tozuka, Y.; Ito, M.; Takeuchi, H. Liposomal diclofenac eye drop formulations targeting the retina: Formulation stability improvement using surface modification of liposomes. Int. J. Pharm. 2012, 436, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Elmaradny, H.A.; Samaha, M.W. Mucoadhesive liposomes as ocular delivery system: Physical, microbiological, and in vivo assessment. Drug Dev. Ind. Pharm. 2010, 36, 108–118. [Google Scholar] [CrossRef] [PubMed]
- De Sá, F.; Taveira, S.F.; Gelfuso, G.M.; Lima, E.M.; Gratieri, T. Liposomal voriconazole (VOR) formulation for improved ocular delivery. Colloids Surf. B Biointerfaces 2015, 133, 331–338. [Google Scholar] [CrossRef]
- Kaur, I.P.; Aggarwal, D.; Singh, H.; Kakkar, S. Improved ocular absorption kinetics of timolol maleate loaded into a bioadhesive niosomal delivery system. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 1467–1472. [Google Scholar] [CrossRef]
- Abdelbary, G.; El-Gendy, N. Niosome-Encapsulated Gentamicin for Ophthalmic Controlled Delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef]
- Duxfield, L.; Sultana, R.; Wang, R.; Englebretsen, V.; Deo, S.; Swift, S.; Rupenthal, I.D.; Al-Kassas, R. Development of gatifloxacin-loaded cationic polymeric nanoparticles for ocular drug delivery. Pharm. Dev. Technol. 2016, 21, 172–179. [Google Scholar] [CrossRef]
- García, M.L.; Pérez, Y.; Gómara, M.J.; Vasconcelos, A.; Vega, E.; Haro, I. Conjugation of cell-penetrating peptides with poly(lactic-co-glycolic acid)-polyethylene glycol nanoparticles improves ocular drug delivery. Int. J. Nanomed. 2015, 10, 609–631. [Google Scholar] [CrossRef] [Green Version]
- Fathalla, Z.M.A.; Khaled, K.A.; Hussein, A.K.; Alany, R.G.; Vangala, A. Formulation and corneal permeation of ketorolac tromethamine-loaded chitosan nanoparticles. Drug Dev. Ind. Pharm. 2015, 42, 514–524. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and evaluation of naringenin-loaded sulfobutylether-β-cyclodextrin/chitosan nanoparticles for ocular drug delivery. Carbohydr. Polym. 2016, 149, 224–230. [Google Scholar] [CrossRef]
- Özsoy, Y.; Güngör, S.; Kahraman, E.; Durgun, M.E. Polymeric micelles as a novel carrier for ocular drug delivery. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–117. [Google Scholar]
- Luschmann, C.; Herrmann, W.; Strauß, O.; Luschmann, K.; Goepferich, A.M. Ocular delivery systems for poorly soluble drugs: An in-vivo evaluation. Int. J. Pharm. 2013, 455, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, Y.; Yang, Z.; Li, M.; Li, F.; Cui, F.; Liu, T.; Shi, W.; Wu, X. Nanomicelle formulation for topical delivery of cyclosporine A into the cornea: In vitro mechanism and in vivo permeation evaluation. Sci. Rep. 2015, 5, 12968. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Rivera, F.; Fernández-Villanueva, D.; Concheiro, A.; Diaz-Rodriguez, P. α-Lipoic Acid in Soluplus® Polymeric Nanomicelles for Ocular Treatment of Diabetes-Associated Corneal Diseases. J. Pharm. Sci. 2016, 105, 2855–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boddu, S.H.S.; Jwala, J.; Chowdhury, M.R.; Mitra, A.K. In Vitro Evaluation of a Targeted and Sustained Release System for Retinoblastoma Cells Using Doxorubicin as a Model Drug. J. Ocul. Pharmacol. Ther. 2010, 26, 459–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holden, C.A.; Tyagi, P.; Thakur, A.; Kadam, R.; Jadhav, G.; Kompella, U.B.; Yang, H. Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 776–783. [Google Scholar] [CrossRef]
- Yavuz, B.; Pehlivan, S.B.; Vural, I.; Ünlü, N. In Vitro/In Vivo Evaluation of Dexamethasone—PAMAM Dendrimer Complexes for Retinal Drug Delivery. J. Pharm. Sci. 2015, 104, 3814–3823. [Google Scholar] [CrossRef]
- Lancina, M.G.; Singh, S.; Kompella, U.B.; Husain, S.; Yang, H. Fast Dissolving Dendrimer Nanofiber Mats as Alternative to Eye Drops for More Efficient Antiglaucoma Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 1861–1868. [Google Scholar] [CrossRef]
- Bravo-Osuna, I.; Vicario-De-La-Torre, M.; Andrés-Guerrero, V.; Sánchez-Nieves, J.; Guzman-Navarro, M.; De La Mata, F.J.; Gómez, R.; Heras, B.D.L.; Argüeso, P.; Ponchel, G.; et al. Novel Water-Soluble Mucoadhesive Carbosilane Dendrimers for Ocular Administration. Mol. Pharm. 2016, 13, 2966–2976. [Google Scholar] [CrossRef]
- Sepahvandi, A.; Eskandari, M.; Moztarzadeh, F. Drug Delivery Systems to the Posterior Segment of the Eye: Implants and Nanoparticles. BioNanoScience 2016, 6, 276–283. [Google Scholar] [CrossRef]
- Falavarjani, K.G. Implantable Posterior Segment Drug Delivery Devices; Novel Alternatives to Currently Available Treatments. J. Ophthalmic Vis. Res. 2009, 4, 191–193. [Google Scholar]
- Wang, X.; Wang, S.; Zhang, Y. Advance of the application of nano-controlled release system in ophthalmic drug delivery. Drug Deliv. 2016, 23, 2897–2901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jervis, L. A Summary of Recent Advances in Ocular Inserts and Implants. J. Bioequiv. Bioavailab. 2017, 9, 320–323. [Google Scholar] [CrossRef]
- Davis, B.; Normando, E.M.; Guo, L.; Turner, L.A.; Nizari, S.; O’Shea, P.; Moss, S.E.; Somavarapu, S.; Cordeiro, M.F. Topical Delivery of Avastin to the Posterior Segment of the Eye In Vivo Using Annexin A5-associated Liposomes. Small 2014, 10, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.K.; Verma, A.; Prajapati, S.K.; Pandey, H.; Pandey, A.C. In vitro, in vivo and pharmacokinetic assessment of amikacin sulphate laden polymeric nanoparticles meant for controlled ocular drug delivery. Appl. Nanosci. 2014, 5, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Li, J.; Wang, J.; Yin, Z.; Zhu, Y.; Liu, W. Development of Timolol-Loaded Galactosylated Chitosan Nanoparticles and Evaluation of Their Potential for Ocular Drug Delivery. AAPS PharmSciTech 2017, 18, 997–1008. [Google Scholar] [CrossRef]
- Yuan, X.; Yuan, Y.-B.; Jiang, W.; Liu, J.; Tian, E.-J.; Shun, H.-M.; Huang, D.; Yuan, X.-Y.; Li, H.; Sheng, J. Preparation of rapamycin-loaded chitosan/PLA nanoparticles for immunosuppression in corneal transplantation. Int. J. Pharm. 2008, 349, 241–248. [Google Scholar] [CrossRef]
- Mohanty, B.; Majumdar, D.K.; Mishra, S.K.; Panda, A.K.; Patnaik, S. Development and characterization of itraconazole-loaded solid lipid nanoparticles for ocular delivery. Pharm. Dev. Technol. 2014, 20, 458–464. [Google Scholar] [CrossRef]
- Pokharkar, V.; Patil, V.; Mandpe, L. Engineering of polymer–surfactant nanoparticles of doxycycline hydrochloride for ocular drug delivery. Drug Deliv. 2015, 22, 955–968. [Google Scholar] [CrossRef] [Green Version]
- Yingfang, F.; Zhuang, B.; Wang, C.; Xu, X.; Xu, W.; Lv, Z.-H. Pimecrolimus micelle exhibits excellent therapeutic effect for Keratoconjunctivitis Sicca. Colloids Surf. B Biointerfaces 2016, 140, 1–10. [Google Scholar] [CrossRef]
- Sah, A.K.; Suresh, P.K.; Verma, V.K. PLGA nanoparticles for ocular delivery of loteprednol etabonate: A corneal penetration study. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1156–1164. [Google Scholar] [CrossRef] [Green Version]
- Abla, M.J.; Singh, N.D.; Banga, A.K. Role of Nanotechnology in Skin Delivery of Drugs. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–13. [Google Scholar]
- Jahromi, M.A.M.; Zangabad, P.S.; Basri, S.M.M.; Zangabad, K.S.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin. Drug Deliv. 2012, 9, 783–804. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.S.; Mital, N.; Kaur, G.; Singh, D. Topical drug delivery systems: A patent review. Expert Opin. Ther. Pat. 2016, 26, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Piemi, M.P.Y.; Korner, D.; Benita, S.; Marty, J.-P. Positively and negatively charged submicron emulsions for enhanced topical delivery of antifungal drugs. J. Control. Release 1999, 58, 177–187. [Google Scholar] [CrossRef]
- Firooz, A.; Nafisi, S.; Maibach, H.I. Novel drug delivery strategies for improving econazole antifungal action. Int. J. Pharm. 2015, 495, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, I.; Azizoğlu, E.; Ozyazici, M.; Nenni, M.; Gurer-Orhan, H.; Ozbal, S.; Tekmen, I.; Ertam, I.; Unal, I.; Ozer, O. Effective topical delivery systems for corticosteroids: Dermatological and histological evaluations. Drug Deliv. 2016, 23, 1502–1513. [Google Scholar] [CrossRef] [Green Version]
- Desmet, E.; Bracke, S.; Forier, K.; Taevernier, L.; Stuart, M.C.A.; De Spiegeleer, B.; Raemdonck, K.; Van Gele, M.; Lambert, J. An elastic liposomal formulation for RNAi-based topical treatment of skin disorders: Proof-of-concept in the treatment of psoriasis. Int. J. Pharm. 2016, 500, 268–274. [Google Scholar] [CrossRef]
- Cosco, D.; Paolino, D.; Maiuolo, J.; Di Marzio, L.; Carafa, M.; Ventura, C.A.; Fresta, M. Ultradeformable liposomes as multidrug carrier of resveratrol and 5-fluorouracil for their topical delivery. Int. J. Pharm. 2015, 489, 1–10. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Singh, M.R. Development characterization and skin permeating potential of lipid based novel delivery system for topical treatment of psoriasis. Chem. Phys. Lipids 2015, 186, 9–16. [Google Scholar] [CrossRef]
- Shrotriya, S.N.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1471–1482. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Sun, L.; Wang, L.; Lin, Z.; Liu, Z.; Xi, L.; Wang, Z.; Zheng, Y. Loading of water-insoluble celastrol into niosome hydrogels for improved topical permeation and anti-psoriasis activity. Colloids Surf. B Biointerfaces 2019, 182, 110352. [Google Scholar] [CrossRef] [PubMed]
- Goyal, G.; Garg, T.; Malik, B.; Chauhan, G.; Rath, G.; Goyal, A.K. Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv. 2015, 22, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Bragagni, M.; Scozzafava, A.; Mastrolorenzo, A.; Supuran, C.T.; Mura, P. Development and ex vivo evaluation of 5-aminolevulinic acid-loaded niosomal formulations for topical photodynamic therapy. Int. J. Pharm. 2015, 494, 258–263. [Google Scholar] [CrossRef] [PubMed]
- El-Say, K.M.; Abd-Allah, F.I.; Lila, A.E.; Hassan, A.E.-S.A.; Kassem, A.E.A. Diacerein niosomal gel for topical delivery: Development, in vitro and in vivo assessment. J. Liposome Res. 2015, 26, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.-L.; Fan, Z.-L.; Yuan, J.-D.; Chen, P.-P.; Yang, J.-J.; Xu, J.; Zhuge, D.-L.; Jin, B.-H.; Zhu, Q.-Y.; Shen, B.-X.; et al. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf. B Biointerfaces 2017, 160, 704–714. [Google Scholar] [CrossRef]
- Ramezanli, T.; Zhang, Z.; Michniak-Kohn, B.B. Development and characterization of polymeric nanoparticle-based formulation of adapalene for topical acne therapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 143–152. [Google Scholar] [CrossRef]
- Balzus, B.; Sahle, F.F.; Hönzke, S.; Gerecke, C.; Schumacher, F.; Hedtrich, S.; Kleuser, B.; Bodmeier, R. Formulation and ex vivo evaluation of polymeric nanoparticles for controlled delivery of corticosteroids to the skin and the corneal epithelium. Eur. J. Pharm. Biopharm. 2017, 115, 122–130. [Google Scholar] [CrossRef]
- Abd-Elsalam, W.H.; El-Zahaby, S.A.; Al-Mahallawi, A.M. Formulation and in vivo assessment of terconazole-loaded polymeric mixed micelles enriched with Cremophor EL as dual functioning mediator for augmenting physical stability and skin delivery. Drug Deliv. 2018, 25, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Kandekar, S.G.; Singhal, M.; Sonaje, K.B.; Kalia, Y.N. Polymeric micelle nanocarriers for targeted epidermal delivery of the hedgehog pathway inhibitor vismodegib: Formulation development and cutaneous biodistribution in human skin. Expert Opin. Drug Deliv. 2019, 16, 667–674. [Google Scholar] [CrossRef]
- Ramezanli, T.; Kilfoyle, B.E.; Zhang, Z.; Michniak-Kohn, B.B. Polymeric nanospheres for topical delivery of vitamin D3. Int. J. Pharm. 2017, 516, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Lapteva, M.; Mignot, M.; Mondon, K.; Möller, M.; Gurny, R.; Kalia, Y.N. Self-assembled mPEG-hexPLA polymeric nanocarriers for the targeted cutaneous delivery of imiquimod. Eur. J. Pharm. Biopharm. 2019, 142, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Beh, C.C.; Mammucari, R.; Foster, N.R. Lipids-based drug carrier systems by dense gas technology: A review. Chem. Eng. J. 2012, 188, 1–14. [Google Scholar] [CrossRef]
- Verma, D.; Verma, S.; Blume, G.; Fahr, A. Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: A skin penetration and confocal laser scanning microscopy study. Eur. J. Pharm. Biopharm. 2003, 55, 271–277. [Google Scholar] [CrossRef]
- Shah, K.A.; Date, A.; Joshi, M.; Patravale, V.B. Solid lipid nanoparticles (SLN) of tretinoin: Potential in topical delivery. Int. J. Pharm. 2007, 345, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Şenyiğit, T.; Sonvico, F.; Barbieri, S.; Ozer, O.; Santi, P.; Colombo, P. Lecithin/chitosan nanoparticles of clobetasol-17-propionate capable of accumulation in pig skin. J. Control. Release 2010, 142, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Katiyar, S.S.; Jain, S.; Jain, S. Nanoemulsion loaded gel for topical co-delivery of clobitasol propionate and calcipotriol in psoriasis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1473–1482. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, K.; Paul, S.; Singh, S.; Singh, S.; Jain, S.K. A Mechanistic Study to Determine the Structural Similarities between Artificial Membrane Strat-M™ and Biological Membranes and Its Application to Carry Out Skin Permeation Study of Amphotericin B Nanoformulations. AAPS PharmSciTech 2018, 19, 1606–1624. [Google Scholar] [CrossRef]
- Shah, P.P.; Desai, P.R.; Patel, A.R.; Singh, M. Skin permeating nanogel for the cutaneous co-delivery of two anti-inflammatory drugs. Biomaterials 2012, 33, 1607–1617. [Google Scholar] [CrossRef] [Green Version]
- Hasanovic, A.; Zehl, M.; Reznicek, G.; Valenta, C. Chitosan-tripolyphosphate nanoparticles as a possible skin drug delivery system for aciclovir with enhanced stability. J. Pharm. Pharmacol. 2009, 61, 1609–1616. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Buheazaha, T.M.; Al-Homoud, H.S.; Al-Nasif, H.A.; Sarafroz; Mohammed, T. A comparative ex vivo permeation evaluation of a novel 5-Fluorocuracil nanoemulsion-gel by topically applied in the different excised rat, goat, and cow skin. Saudi J. Biol. Sci. 2020, 27, 1024–1040. [Google Scholar] [CrossRef]
- Jain, S.; Addan, R.; Jain, S.; Harde, H.; Mahajan, R.R. Comparative assessment of efficacy and safety potential of multifarious lipid based Tacrolimus loaded nanoformulations. Int. J. Pharm. 2019, 562, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Somagoni, J.; Boakye, C.H.A.; Godugu, C.; Patel, A.R.; Faria, H.A.M.; Zucolotto, V.; Singh, M. Nanomiemgel—A Novel Drug Delivery System for Topical Application—In Vitro and In Vivo Evaluation. PLoS ONE 2014, 9, e115952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, Z. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur. J. Pharm. Biopharm. 2003, 56, 189–196. [Google Scholar] [CrossRef]
- Kong, X.; Zhao, Y.; Quan, P.; Fang, L. Development of a topical ointment of betamethasone dipropionate loaded nanostructured lipid carrier. Asian J. Pharm. Sci. 2016, 11, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Hafeez, A.; Kazmi, I. Dacarbazine nanoparticle topical delivery system for the treatment of melanoma. Sci. Rep. 2017, 7, 16517. [Google Scholar] [CrossRef] [Green Version]
- Abdelbary, A.A.; AbouGhaly, M.H. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: Application of Box–Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int. J. Pharm. 2015, 485, 235–243. [Google Scholar] [CrossRef]
- Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.; Igartua, M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1551–1573. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.; Igartua, M. Nanotechnology approaches for skin wound regeneration using drug-delivery systems. In Nanobiomaterials in Soft Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 31–55. [Google Scholar]
- Chu, Y.; Yu, D.; Wang, P.; Xu, J.; Li, D.; Ding, M. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen. 2010, 18, 499–505. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Her, C.-H.; Comune, M.; Moia, C.; Lopes, A.; Porporato, P.E.; Vanacker, J.; Lam, M.C.; Steinstraesser, L.; Sonveaux, P.; et al. PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing. J. Control. Release 2014, 194, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Mathew, A.P.; Sosnik, A. Metal Oxide Nanoparticles as Versatile Therapeutic Agents Modulating Cell Signaling Pathways: Linking Nanotechnology with Molecular Medicine. Appl. Mater. Today 2017, 7, 91–103. [Google Scholar] [CrossRef]
- Sankar, R.; Dhivya, R.; Shivashangari, K.S.; Ravikumar, V. Wound healing activity of Origanum vulgare engineered titanium dioxide nanoparticles in Wistar Albino rats. J. Mater. Sci. Mater. Electron. 2014, 25, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A.; et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomed. Nanotechnol. Biol. Med. 2014, 11, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Gainza, G.; Pastor, M.; Aguirre, J.J.; Villullas, S.; Pedraz, J.L.; Hernández, R.M.; Igartua, M. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: In vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J. Control. Release 2014, 185, 51–61. [Google Scholar] [CrossRef]
- Olekson, M.; Faulknor, R.; Bandekar, A.; Sempkowski, M.; Hsia, H.; Berthiaume, F. SDF-1 liposomes promote sustained cell proliferation in mouse diabetic wounds. Wound Repair Regen. 2015, 23, 711–723. [Google Scholar] [CrossRef]
- Fukui, T.; Kawaguchi, A.T.; Takekoshi, S.; Miyasaka, M.; Sumiyoshi, H.; Tanaka, R. Liposome-Encapsulated Hemoglobin Accelerates Skin Wound Healing in Diabetic dB/dB Mice. Artif. Organs 2017, 33, 146–326. [Google Scholar] [CrossRef]
- Li, X.; Nan, K.; Li, L.; Zhang, Z.; Chen, H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly(ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr. Polym. 2012, 88, 84–90. [Google Scholar] [CrossRef]
- Bairagi, U.; Mittal, P.; Singh, J.; Mishra, B. Preparation, characterization, and in vivo evaluation of nano formulations of ferulic acid in diabetic wound healing. Drug Dev. Ind. Pharm. 2018, 44, 1783–1796. [Google Scholar] [CrossRef]
- Raguvaran, R.; Manuja, B.K.; Chopra, M.; Thakur, R.; Anand, T.; Kalia, A.; Manuja, A. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int. J. Biol. Macromol. 2017, 96, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Volova, T.; Oluwafemi, O.S.; Rajeshkumar, S.; Thomas, S.; Kalarikkal, N. Nano formulated proanthocyanidins as an effective wound healing component. Mater. Sci. Eng. C 2020, 106, 110056. [Google Scholar] [CrossRef]
- Lau, P.; Bidin, N.; Islam, S.; Shukri, W.N.B.W.M.; Zakaria, N.; Musa, N.; Krishnan, G. Influence of gold nanoparticles on wound healing treatment in rat model: Photobiomodulation therapy. Lasers Surg. Med. 2016, 49, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, K.K.; Lopes, A.; Koussoroplis, S.; Payen, V.; Moia, C.; Zhu, H.; Sonveaux, P.; Carmeliet, P.; Rieux, A.D.; Vandermeulen, G.; et al. Combined effects of PLGA and vascular endothelial growth factor promote the healing of non-diabetic and diabetic wounds. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; Chamberlain, L.; Faxius, L.; Johnston, G.W.; Jin, S.; Bjursten, L.M. Soft tissue response to titanium dioxide nanotube modified implants. Acta Biomater. 2011, 7, 3209–3215. [Google Scholar] [CrossRef]
- Bryington, M.S.; Hayashi, M.; Kozai, Y.; Van DeWeghe, S.; Andersson, M.; Wennerberg, A.; Jimbo, R. The influence of nano hydroxyapatite coating on osseointegration after extended healing periods. Dent. Mater. 2013, 29, 514–520. [Google Scholar] [CrossRef]
- Khosravi, N.; Maeda, A.; Dacosta, R.S.; Davies, J.E. Nanosurfaces modulate the mechanism of peri-implant endosseous healing by regulating neovascular morphogenesis. Commun. Biol. 2018, 1, 72. [Google Scholar] [CrossRef] [Green Version]
- Hashempour, S.; Ghanbarzadeh, S.; Maibach, H.I.; Ghorbani, M.; Hamishehkar, H. Skin toxicity of topically applied nanoparticles. Ther. Deliv. 2019, 10, 383–396. [Google Scholar] [CrossRef]
- Samberg, M.E.; Oldenburg, S.J.; Monteiro-Riviere, N.A. Evaluation of Silver Nanoparticle Toxicity in Skin in Vivo and Keratinocytes in Vitro. Environ. Health Perspect. 2009, 118, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Paddle-Ledinek, J.E.; Nasa, Z.; Cleland, H.J. Effect of Different Wound Dressings on Cell Viability and Proliferation. Plast. Reconstr. Surg. 2006, 117, 110S–118S. [Google Scholar] [CrossRef]
- Program, N.T. NTP Toxicology and Carcinogenesis Studies of Hydroquinone (CAS No. 123-31-9) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1989, 366, 1. [Google Scholar]
- Doktorovová, S.; Kovačević, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Tehranchinia, Z.; Saghi, B.; Rahimi, H. Evaluation of Therapeutic Efficacy and Safety of Tranexamic Acid Local Infiltration in Combination with Topical 4% Hydroquinone Cream Compared to Topical 4% Hydroquinone Cream Alone in Patients with Melasma: A Split-Face Study. Dermatol. Res. Pract. 2018, 2018, 8350317. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Gordo, M.J.; Matji, A.; González, S.; Lalatsa, A.; Torrado, J.J. Tuning the Transdermal Delivery of Hydroquinone upon Formulation with Novel Permeation Enhancers. Pharmaceutics 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Liu, W.; Xue, C.; Zhou, S.; Lan, F.; Bi, L.; Xu, H.; Yang, X.; Zeng, F.-D. Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol. Lett. 2009, 191, 1–8. [Google Scholar] [CrossRef]
- Trop, M.; Novak, M.; Rödl, S.; Hellbom, B.; Kroell, W.; Goessler, W. Silver-Coated Dressing Acticoat Caused Raised Liver Enzymes and Argyria-like Symptoms in Burn Patient. J. Trauma Acute Care Surg. 2006, 60, 648–652. [Google Scholar] [CrossRef]





| S. No | Formulations | Drug | Ailment | In Vivo/Ex Vivo Study | Inference | Reference |
|---|---|---|---|---|---|---|
| 1. | Liposomes | Voriconazole | Fungal keratitis | Ex vivo static permeation through porcine cornea | Voriconazole liposomes made of soyphosphatidylcholine were successful in delivering drug through the cornea for treating fungal keratitis with no irritation in eyes reported. | [38] |
| 2. | Annexin A5-associated liposomes | Bevacizumab | Posterior segment ocular diseases such as glaucoma or neovascular age-related macular degeneration (AMD) | In vivo assays performed in rat eye and rabbit retina | Annexin A5 (AnxA5) is a calcium-dependent phospholipid binding protein whose addition to phospholipid vesicles(PLVs) was found to significantly increase the concentration of encapsulated Avastin (bevacizumab) reaching the posterior segment of the rat eye when compared to an equivalent concentration of PLVs in the absence of AnxA5, or a higher concentration. | [58] |
| 3. | Dendrimers | Dexamethasone | Diabetic retinopathy | In vivo ocular distribution study in Sprague-Dawley rats | Topical delivery of dexamethasone-PAMAM dendrimers increased ocular bioavailability and resulted in increased concentration of the drug in retina. | [51] |
| 4. | Dendrimer hydrogel | Brimonidine and timolol maleate | Glaucoma | Ex vivo studies in freshly excised bovine eyes | Brimonidine and timolol maleate were encapsulated into dendrimers hydrogel (DH) and it was found that the transport of the drugs across the bovine corneal endothelium was significantly increased as compared to the eye drop solution. | [50] |
| 5. | Polymeric nanoparticles | Amikacin | Staphylococcus aureus infection | In vivo studies male New Zealand albino rabbits | The polymeric nanoparticles prepared using nano emulsification method showed controlled release of the drug as compared to the commercial eye drop. The formulation was stable and did not show any irritation on application. | [59] |
| 6. | Galactosylated chitosan nanoparticles | Timolol maleate | Glaucoma | In vivo pharmacodynamic studies in New Zealand albino rabbits | This formulation improved the drug permeation through cornea. The in vivo pharmacodynamic study showed that the formulation substantially improved the drug efficacy and improved its bioavailability. | [60] |
| 7. | Chitosan/PLA nanoparticles | Rapamycin | Immunosuppression in corneal transplantation | In vivo studies in New Zealand rabbits | Chitosan/PLA nanoparticles showed better retention properties at the precorneal site as compared with rapamycin aqueous suspension. The nanoparticles showed an excellent immunosuppressive effect compared with the rapamycin eye drops. | [61] |
| 8. | Eudragit RS100 and RL100 polymeric nanoparticles | Gatifloxacin | Ocular infections such as conjunctivitis, keratitis, and endophthalmitis | Tested on bacteria | The Gatifloxacin-loaded nanoparticles were shown to improve bioavailability by prolonging the retention of the drug in the eyes. | [41] |
| 9. | Solid lipid nanoparticles | Itraconazole | Fungal corneal infections | In vitro studies with goat cornea | Itraconazole solid lipid nanoparticles were prepared using stearic acid and palmitic acid. It was concluded that permeation of itraconazole from stearic acid SLNs was higher than palmitic acid SLNs. The formulation showed better antimicrobial efficacy. | [62] |
| 10. | Gellan gum polymeric nanoparticles | Doxycycline | Corneal neovascularization, recurrent epithelial erosions and sterile corneal ulcerations | Eye irritancy test in male New Zealand albino rabbits | Doxycycline nanoparticles showed sustained release of the drug with no irritant properties. Antibacterial studies showed that the formulation inhibited bacterial growth at very low concentrations than that of the pure drug. | [63] |
| 11. | Chitosan nanoparticles | Ketorolac tromethamine | Post-operative eye inflammation | Ex vivo permeation studies with cornea obtained from porcine eye balls | The in vitro release study performed suggested that the prepared formulation is capable of sustaining drug release over a period of 6 h as compared to ketorolac tromethamine solution that releases the drug rapidly over a period of 3 h. | [43] |
| 12. | Nanomicelles | Pimecrolimus | Keratoconjunctivitis Sicca | In vivo test in Kunming (KM) mice | The formulation resulted in higher drug encapsulation capability with drug loading and encapsulation efficiency of 7.57% ± 0.10% and 97.9% ± 1.26%, respectively. It was found that the nanocarrier protects the eyes from drug-induced toxicity and vision loss. The prepared nanomicelar formulation inhibits the cytokine production and shows a significantly increased healing process as compared to the other group. | [64] |
| 13. | Niosome | Gentamicin | Ocular infections | In vivo Ocular irritancy test performed on albino rabbits | The in vitro evaluation of gentamicin niosomes showed that the niosomes made of tween 60, cholesterol and diacetyl phosphate prolonged the release of the drug as compared to the gentamicin solution. | [40] |
| 14. | PLGA nanoparticles | Loteprednol etabonate | Ocular inflammation | Ex vivo transcorneal permeation profile of optimized PLGA nanoparticle formulation was assessed on excised goat cornea | The prepared formulation showed better penetration of the drug across excised goat cornea and adhered to the ocular surface for a prolonged period as compared to the pure drug suspension. | [65] |
| S. No. | Product | Drug | Formulation | Application | Current Status | References |
|---|---|---|---|---|---|---|
| 1. | Dermos™ | Paclitaxel | Nanosomal formulation with diameter less than 1 nm | AIDS-associated Kaposi’s sarcoma | On the market | [66] |
| 2. | Estrasorb | Estradiol hemihydrate | Micellar nanoparticles, an emulsion of estrogen and soybean oil | Prevention of hot flushes and treatment of vasomotor symptoms associated with menopause | On the market (FDA approval) | [88] |
| 3. | Pevaryllipogel | Econazole | Liposomes | Anti-fungal | On the market | [89] |
| 4. | NB-001 | -- | NanoStat™ topical formulation technique for specifically targeting microbes | Treatment of cold sores associated with herpes labialis | Clinical trial | [66] |
| S. no. | Nano Formulation | Drug | Ailment/Disease | In-Vivo/Ex-Vivo Model | Results | References |
|---|---|---|---|---|---|---|
| 1. | Solid lipid nanoparticles | Tretinoin | Psoriasis, acne, photoaging and epithelial skin cancer | Ex vivo permeation and irritation study using whister rats. | Prepared formulation improved photo stability and toleratability, reduced irritation, and increased drug permeation as compared to the free drug. | [90] |
| 2. | Polymeric nanoparticles (Lecithin-Chitosan) | Clobetasol-17-propionate | Inflammatory skin diseases | In vitro permeation study through franz diffusion cell. | Polymeric nanoparticles reduced side effects of the drug as compared to the marketed creams. Prepared nanoparticles increased epidermal targeting. | [91] |
| 3. | Nano emulsion-based gel | Clobitasol propionate and calcipotriol | Psoriasis | Ex vivo permeation study is performed using pig ear skin. In vivo efficacy was performed using BALB/c model. | Optimized formulation showed higher anti-psoriatic activity as compared to the free drug. | [92] |
| 4. | Nanoethogel and nanogel formulations | Amphotericin B | Dermatophytes and surface fungal infections | Ex vivo permeation study through rat skin and porcine ear skin | It was found that Strat-M™ is a better alternative to carry out skin permeation experiments due to the consistent results, reproducibility, easy availability. | [93] |
| 5. | Nanogel | Spantide II and ketoprofen | Allergic contact dermatitis and psoriasis | Ex vivo permeation study was performed using human skin psoriatic plaque like model was developed on C57BL/6 mice | Deposition of drugs was increased 8.5- and 9.5-fold in dermis and epidermis, respectively, as compared to the free drug. Prepared formulation deposited drugs in deeper tissues. | [94] |
| 6. | Chitosan–tripolyphosphate nanoparticles | Aciclovir | Herpes infections | In vitro permeation studies with porcine abdominal skin | Incorporation of aciclovir into chitosan-tripolyphosphate nanoparticles significantly improves its chemical stability. Nanoparticle formulation improved permeation as compared to the free drug. | [95] |
| 7. | Nano emulsion-gel | 5-Fluorouracil | Actinic keratosis and Non-melanoma skin cancers | Ex vivo permeation study using rat, goat and cow skin. | It was found that prepared nanoemulsion gel increased permeation by 1.2 fold in rat skin and 12.51 in the goat skin. Prepared formulation was safer compared to the free drug. | [96] |
| 8. | Solid lipid nanoparticle and Nanostructure lipid carrier | Tacrolimus | Psoriasis | Ex vivo permeation study through pig ear skin Anti-psoriasis was model developed using mice model | Tac liquid crystal nanoparticle (LCNP) Tac-SLN, Tac-NLC and Tac-liposome-loaded gels showed 14-, 11.5-, 12.5- and 3.7-fold increments in dermal bioavailability respectively, in comparison to free Tac-loaded gel. | [97] |
| 9. | Nanoemulgel | Aceclofenac and capsaicin | Psoriasis | Ex vivo permeation study was performed using human skin | Nanoemulgel showed a controlled release drug pattern as compared to free drug. It was also found that prepared formulation showed 2.02- and 1.97-fold higher permeation as compared to their respective free drugs. | [98] |
| 10 | Solid lipid particles and nano emulsion | Triptolide | Anti-inflammatory | carrageenan-induced inflamation model was developed using wistar rats | Improved availability of drug at target size, reduced side effect like irritation and staining. | [99] |
| 11 | Nanostructured lipid carrier | Betamethasone | Atopic dermatitis | Ex vivo permeation study was performed using rabbit skin | Drug-loaded lipid carrier showed high retention as compared to the free drugs. | [100] |
| 12 | Nanoparticle delivery | Dacarbazine | Melanoma | In vitro permeation study through franz diffusion cell | Rate of drug release was higher in nanoparticles as compared to the suspension of the drug. | [101] |
| 13 | Niosomes | Methotrexate | Psoriasis | In vivo skin deposition study using wistar rats | Results showed that targeted MTX delivery might be achieved using topically applied niosomes for enhanced treatment of psoriasis. | [102] |
| S. No. | Nano Formulation | Drug | Inferences | Reference |
|---|---|---|---|---|
| 1. | Methoxy poly(ethylene glycol)-graft-chitosan (-mPEG) film | Curcumin | Applied in full-thickness punch wounds model of SD (Sprague dawley) rats showed faster wound reduction and shortened re-epithelialization period as compared to the MPEG-chitosan film. Masson’s trichrome staining indicated that the wound treated with curcumin-MPEG-chitosan film had a compact and well-aligned collagen as compared MPEG-chitosan film treated wound. | [115] |
| 2. | Poly (lactic-co-glycolic acid) nanoparticles | Ferulic acid (FA) | In vivo studies showed that FA nanoparticles applied topically hydrogel and administrated orally (dispersion) promoted wound healing in diabetic rats. | [116] |
| 3. | Nanoparticles incorporated in hydrogel | Zinc oxide | ZnO causes toxicity to the fibroblast cells at higher concentration. Hydrogel containing 4.98% of ZnO nanoparticles showed complete monolayer formation. | [117] |
| 4. | Liposomes prepared using DSPC and DSPA and cholesterol | Stromal cell-derived factor-1 (SDF-1) | The SDF-1 liposomes maintained and promoted sustained proliferation of SDF-1 in the wound that led to positive effect on wound closure. | [113] |
| 5. | Soluplus nanodispersion | Proanthocyanidins | The formulation showed antibacterial activity against E. coli, S. aureus and Bacillus. The bacterial cell membrane became more permeable and the cell structure was disrupted. It was found that this formulation could improve wound healing without forming scars. The histopathological assay of the treated animals showed complete re-epithelialisation, migration of cells, proliferation of cells and fibroblast attachment. | [118] |
| 6. | Gold nanoparticles | Gold | Histological examinations showed that gold nanoparticles in photo biomodulation therapy were found to be more effective in contracting and accelerating wound healing due to enhanced epithelialization, collagen deposition and fast vascularization. | [119] |
| 7. | Chitosan PEG and tetramethyl orthosilicate nanoparticles | Curcumin | The formulation can be used to treat the burn wounds efficiently reducing bacterial load and enhancing wound healing. | [111] |
| 8. | Solid lipid nanoparticles (5% (w/v) Precirol® 133 ATO 5) and nanolipid carrier (Precirol® 146 ATO 5 and 20 mg of Miglyol® 182) (SLN and NLC) | Recombinant human epidermal growth factor (rhEGF) | The bioactivity of the solid lipid nanoparticles was higher in the cell lines studied as compared to the free rhEGF. | [112] |
| 9. | PLGA nanoparticles | Vascular endothelial growth factor (VEGF) | The VEGF released from the PLGA-VEGF nanoparticles induced neovascularization significantly and did not show any cytotoxicity. It was suggested that the formulation accelerated wound closure by targeting different cells involved in wound healing. | [120] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koppa Raghu, P.; Bansal, K.K.; Thakor, P.; Bhavana, V.; Madan, J.; Rosenholm, J.M.; Mehra, N.K. Evolution of Nanotechnology in Delivering Drugs to Eyes, Skin and Wounds via Topical Route. Pharmaceuticals 2020, 13, 167. https://doi.org/10.3390/ph13080167
Koppa Raghu P, Bansal KK, Thakor P, Bhavana V, Madan J, Rosenholm JM, Mehra NK. Evolution of Nanotechnology in Delivering Drugs to Eyes, Skin and Wounds via Topical Route. Pharmaceuticals. 2020; 13(8):167. https://doi.org/10.3390/ph13080167
Chicago/Turabian StyleKoppa Raghu, Pratheeksha, Kuldeep K. Bansal, Pradip Thakor, Valamla Bhavana, Jitender Madan, Jessica M. Rosenholm, and Neelesh Kumar Mehra. 2020. "Evolution of Nanotechnology in Delivering Drugs to Eyes, Skin and Wounds via Topical Route" Pharmaceuticals 13, no. 8: 167. https://doi.org/10.3390/ph13080167
APA StyleKoppa Raghu, P., Bansal, K. K., Thakor, P., Bhavana, V., Madan, J., Rosenholm, J. M., & Mehra, N. K. (2020). Evolution of Nanotechnology in Delivering Drugs to Eyes, Skin and Wounds via Topical Route. Pharmaceuticals, 13(8), 167. https://doi.org/10.3390/ph13080167