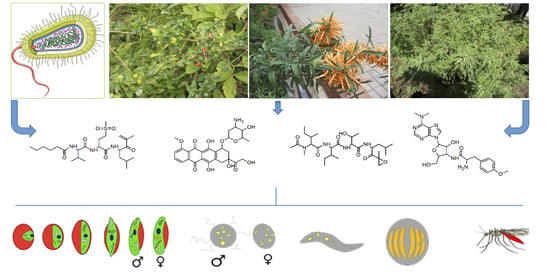
Natural Products: A Potential Source of Malaria Transmission Blocking Drugs?
Abstract
:1. Introduction
1.1. Transmission-Blocking: An Integral Tool for Malaria Elimination
1.2. Can Natural Products Prove a Panacea for Transmission-Blocking Drug Discovery Efforts?
2. Effectiveness of Natural Products Against Transmission-Blocking Stages
2.1. Microbial-Derived Natural Products
2.1.1. Ionophores
2.1.2. Peptides, Glycosides and Miscellaneous
2.1.3. Mycotoxins
| Compound | MW | cLogP | Transmission-Blocking Stage Activity (IC50, µM/% inhibition @ >5 µM a or <0.5 µM b) | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| EG | LG | Mic | Mac | ESS | Ooc | ||||
| Ionophores | |||||||||
| Salinomycin | 751 | 5 | 0.014 | 0.006 | 0.035 | 0.002 c; 0.018 d | [45] | ||
| Nigericin | 724 | 4.69 | 0.003 | 0.001 | [45] | ||||
| Monensin | 670 | 3.74 | 0.002 | 0.006 | 0.017 | 0.002 c; 0.001 d | [45] | ||
| Maduramicin | 934 | 1.47 | 0.015 | 100% e | [48,49] | ||||
| Peptides, glycosides and miscellaneous | |||||||||
| Epoxomicin | 554 | 2.12 | 99.8% a | 0.0004 | Inactive | 0.008 | 100% b | [48,50,51,52,53,54] | |
| Carmaphycin B | 515 | 3.31 | 0.160 | [57] | |||||
| Thiostrepton | 1664 | −1.04 | 2.8 | 1.8 | 0.096 | 1.4 | 8 | Active a | [4,9,59] |
| Dactinomycin | 1255 | 0.6 | 0.015 | [49] | |||||
| Romidepsin | 540 | 1.39 | 0.637 | Active b | [49,60] | ||||
| Adriamycin | 579 | 0.36 | 0.526 | [49] | |||||
| Plicamycin | 1085 | 0.25 | 0.833 | [49] | |||||
| Puromycin | 471 | −0.22 | 0.103 | 0.110 | 100% a | [4,6,7,50] | |||
| Cycloheximide | 281 | 1.3 | 0.6 | 0.477 | 100% a | 100% a | [50,61] | ||
| Chlorotonil A | 479 | 4.81 | 0.030 | [62] | |||||
| Mycotoxins | |||||||||
| P-Orlandin | 410 | 3.18 | 56.7% a; 35.3% a | [66] | |||||
| Aphidicolin | 338 | 2.39 | 100% b | [64] | |||||
2.2. Plant-Derived Natural Products
2.2.1. Terpenes and Terpenoids
2.2.2. Alkaloids, Steroids and Miscellaneous
| Compound | MW | cLogP | Transmission-Blocking Stage Activity (IC50, µM/% inhibition @ > 5 µM a) | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| EG | LG | Mic | Mac | ESS | Ooc | ||||
| Terpenes and Terpenoids | |||||||||
| Artemisinin | 282 | 2.5 | 0.012 | 0.037 | 0.224 | 0.120 | Inactive | 93% a | [4,6,7,9,50,51,63,70,71] |
| 1α,4α-* | 320 | 0.97 | 17.5 | 6.3 | [76] | ||||
| Vernodalol | 392 | 1.45 | 18.7 | [77] | |||||
| Daucovirgolide G | 446 | 3.63 | 82.3 b; 48.4 c | [78,79] | |||||
| Taxol | 853 | 3.39 | ~80% a | [81] | |||||
| Azadirachtin A | 720 | 1.08 | 3.5 | 17.2 | [82,88] | ||||
| Deacetylnimbin | 498 | 2.77 | 6 to 25 | [89] | |||||
| Alkaloids, Steroids and Miscellaneous | |||||||||
| Quinine | 324 | 2.81 | 0.44 | 0.318 | 29% a | 22.6%a | 85%a | [4,5,7,49,50,51,61,63] | |
| Dihydronitidine | 349 | 3.65 | 1.7 | [94] | |||||
| Tryptanthrin | 248 | 2.16 | 95% d | Inactive | [95] | ||||
| Omacetaxine | 545 | 2.47 | 0.083 | [49] | |||||
| Withaferin A | 470 | 3.45 | 0.372 | [49] | |||||
| Lophirone E | 372 | 3.95 | 0.14 | [101] | |||||
2.3. Herbal Remedies as Gametocytocidal Agents
2.4. Endectocidal Activity of Plant Extracts Against Anopheles
2.5. Transmission-Blocking Activities of Synthetic Derivatives of Natural Compound Analogues Currently in Clinical Trials
3. Future Perspectives
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cox, F.E. History of the discovery of the malaria parasites and their vectors. Parasites Vectors 2010, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. World Malaria Report 2019; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Plouffe, D.M.; Wree, M.; Du, A.Y.; Meister, S.; Li, F.; Patra, K.; Lubar, A.; Okitsu, S.L.; Flannery, E.L.; Kato, N.; et al. High-Throughput Assay and Discovery of Small Molecules that Interrupt Malaria Transmission. Cell Host Microbe 2016, 19, 114–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucantoni, L.; Duffy, S.; Adjalley, S.H.; Fidock, D.A.; Avery, V.M. Identification of MMV Malaria Box Inhibitors of Plasmodium falciparum Early-Stage Gametocytes Using a Luciferase-Based High-Throughput Assay. Antimicrob. Agents Chemother. 2013, 57, 6050–6062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucantoni, L.; Fidock, D.A.; Avery, V.M. Luciferase-Based, High-Throughput Assay for Screening and Profiling Transmission-Blocking Compounds against Plasmodium falciparum Gametocytes. Antimicrob. Agents Chemother. 2016, 60, 2097–2107. [Google Scholar] [CrossRef] [Green Version]
- Duffy, S.; Avery, V.M. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar. J. 2013, 12, 408. [Google Scholar] [CrossRef] [Green Version]
- Ruecker, A.; Mathias, D.K.; Straschil, U.; Churcher, T.S.; Dinglasan, R.R.; Leroy, D.; Sinden, R.E.; Delves, M. A Male and Female Gametocyte Functional Viability Assay to Identify Biologically Relevant Malaria Transmission-Blocking Drugs. Antimicrob. Agents Chemother. 2014, 58, 7292–7302. [Google Scholar] [CrossRef] [Green Version]
- Delves, M.; Ruecker, A.; Straschil, U.; Lelièvre, J.; Marques, S.R.L.O.; López-Barragán, M.J.; Herreros, E.; Sinden, R.E. Male and Female Plasmodium falciparum Mature Gametocytes Show Different Responses to Antimalarial Drugs. Antimicrob. Agents Chemother. 2013, 57, 3268–3274. [Google Scholar] [CrossRef] [Green Version]
- Burrows, J.N.; Duparc, S.; Gutteridge, W.E.; van Huijsduijnen, R.H.; Kaszubska, W.; Macintyre, F.; Mazzuri, S.; Möhrle, J.J.; Wells, T.N. New developments in anti-malarial target candidate and product profiles. Malar. J. 2017, 16, 26. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, A.S.; Skinner-Adams, T.S.; Gardiner, D.L.; Trenholme, K.R. Plasmodium falciparum gametocytes: With a view to a kill. Parasitology 2013, 140, 1718–1734. [Google Scholar] [CrossRef] [Green Version]
- Kiszewski, A.E. Blocking Plasmodium falciparum Malaria Transmission with Drugs: The Gametocytocidal and Sporontocidal Properties of Current and Prospective Antimalarials. Pharmaceuticals 2010, 4, 44–68. [Google Scholar] [CrossRef] [Green Version]
- Roberts, L.; Enserink, M. Did they really say… eradication? Science 2007, 318, 1544–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, P.L.; Brown, G.; Arevalo-Herrera, M.; Binka, F.; Chitnis, C.; Collins, F.; Doumbo, O.K.; Greenwood, B.; Hall, B.F.; Levine, M.M. A research agenda to underpin malaria eradication. PLoS Med. 2011, 8, e1000406. [Google Scholar] [CrossRef] [PubMed]
- Leroy, D.; Campo, B.; Ding, X.C.; Burrows, J.N.; Cherbuin, S. Defining the biology component of the drug discovery strategy for malaria eradication. Trends Parasitol. 2014, 30, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Burrows, J.; Slater, H.; Macintyre, F.; Rees, S.; Thomas, A.; Okumu, F.; van Huijsduijnen, R.H.; Duparc, S.; Wells, T.N. A discovery and development roadmap for new endectocidal transmission-blocking agents in malaria. Malar. J. 2018, 17, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shretta, R.; Liu, J.; Cotter, C.; Cohen, J.; Dolenz, C.; Makomva, K.; Newby, G.; Ménard, D.; Phillips, A.; Tatarsky, A.; et al. Malaria Elimination and Eradication. In Major Infectious Diseases, 3rd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017; pp. 315–346. [Google Scholar]
- Killeen, G.F.; Tatarsky, A.; Diabate, A.; Chaccour, C.J.; Marshall, J.M.; Okumu, F.O.; Brunner, S.; Newby, G.; Williams, Y.A.; Malone, D.; et al. Developing an expanded vector control toolbox for malaria elimination. BMJ Glob. Health 2017, 2, e000211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueda-Zubiaurre, A.; Yahiya, S.; Fischer, O.J.; Hu, X.; Saunders, C.N.; Sharma, S.; Straschil, U.; Shen, J.; Tate, E.W.; Delves, M.J.; et al. Structure–Activity Relationship Studies of a Novel Class of Transmission Blocking Antimalarials Targeting Male Gametes. J. Med. Chem. 2019, 63, 2240–2262. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Duong, S.; Tan, B.; Dara, P.; Deng, C.; Sreng, S.; Seila, S.; Ou, F.; Jian, H.; Li, G. Rapid and effective malaria control in Cambodia through mass administration of artemisinin-piperaquine. Malar. J. 2010, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Shekalaghe, S.; Drakeley, C.; Gosling, R.; Ndaro, A.; Van Meegeren, M.; Enevold, A.; Alifrangis, M.; Mosha, F.; Sauerwein, R.; Bousema, T. Primaquine Clears Submicroscopic Plasmodium falciparum Gametocytes that Persist after Treatment with Sulphadoxine-Pyrimethamine and Artesunate. PLoS ONE 2007, 2, e1023. [Google Scholar] [CrossRef] [Green Version]
- Smithuis, F.; Kyaw, M.K.; Phe, O.; Win, T.; Aung, P.P.; Oo, A.P.P.; Naing, A.L.; Nyo, M.Y.; Myint, N.Z.H.; Imwong, M.; et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: An open-label randomised trial. Lancet Infect. Dis. 2010, 10, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Okebe, J.; Bousema, T.; Affara, M.; Di Tanna, G.L.; Dabira, E.; Gaye, A.; Sanya-Isijola, F.; Badji, H.; Correa, S.; Nwakanma, D.; et al. The Gametocytocidal Efficacy of Different Single Doses of Primaquine with Dihydroartemisinin-piperaquine in Asymptomatic Parasite Carriers in The Gambia: A Randomized Controlled Trial. EBioMedicine 2016, 13, 348–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, J.; Allen, E.; Workman, L.; Mabuza, A.; Swanepoel, H.; Malatje, G.; Frean, J.; Wiesner, L.; Barnes, K.I. Safety and tolerability of single low-dose primaquine in a low-intensity transmission area in South Africa: An open-label, randomized controlled trial. Malar. J. 2019, 18, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Guidelines for the Treatment of Malaria; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.N. Natural products as starting points for future anti-malarial therapies: Going back to our roots? Malar. J. 2011, 10, S3. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef]
- Gaillard, T.; Dormoi, J.; Madamet, M.; Pradines, B. Macrolides and associated antibiotics based on similar mechanism of action like lincosamides in malaria. Malar. J. 2016, 15, 85. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, T.; Madamet, M.; Pradines, B. Tetracyclines in malaria. Malar. J. 2015, 14, 445. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wang, Y.; Li, D.; Chen, Y.; Dou, Q.P. Are we seeing a resurgence in the use of natural products for new drug discovery? Expert Opin. Drug Discov. 2019, 14, 417–420. [Google Scholar] [CrossRef]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Meyer, K.J.; Iinishi, A.; Favre-Godal, Q.; Green, R.; Manuse, S.; Caboni, M.; Mori, M.; Niles, S.; Ghiglieri, M.; et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 2019, 576, 459–464. [Google Scholar] [CrossRef] [PubMed]
- D’Incalci, M.; Galmarini, C.M. A Review of Trabectedin (ET-743): A Unique Mechanism of Action. Mol. Cancer Ther. 2010, 9, 2157–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, D.S.; Jain, J.P.; Kangas, M.; Lefèvre, G.; Machineni, S.; Griffin, P.; Lickliter, J. Open-Label, Single-Dose, Parallel-Group Study in Healthy Volunteers To Determine the Drug-Drug Interaction Potential between KAE609 (Cipargamin) and Piperaquine. Antimicrob. Agents Chemother. 2015, 59, 3493–3500. [Google Scholar] [CrossRef] [Green Version]
- Phyo, A.P.; Jittamala, P.; Nosten, F.; Pukrittayakamee, S.; Imwong, M.; White, N.J.; Duparc, S.; MacIntyre, F.; Baker, M.; Möhrle, J.J. Antimalarial activity of artefenomel (OZ439), a novel synthetic antimalarial endoperoxide, in patients with Plasmodium falciparum and Plasmodium vivax malaria: An open-label phase 2 trial. Lancet Infect. Dis. 2016, 16, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Yoo, E.; Schulze, C.J.; Stokes, B.H.; Onguka, O.; Yeo, T.; Mok, S.; Gnädig, N.F.; Zhou, Y.; Kurita, K.; Foe, I.T.; et al. The Antimalarial Natural Product Salinipostin a Identifies Essential α/β Serine Hydrolases Involved in Lipid Metabolism in P. Falciparum Parasites. Cell Chem. Biol. 2020, 27, 143–157. [Google Scholar] [CrossRef]
- Birkholtz, L.M.; Coetzer, T.L.; Mancama, D.; Leroy, D.; Alano, P. Discovering New Transmission-Blocking Antimalarial Compounds: Challenges and Opportunities. Trends parasitol. 2016, 32, 669–681. [Google Scholar] [CrossRef] [Green Version]
- Delves, M.; Miguel-Blanco, C.; Matthews, H.; Molina, I.; Ruecker, A.; Yahiya, S.; Straschil, U.; Abraham, M.; León, M.L.; Fischer, O.; et al. A high throughput screen for next-generation leads targeting malaria parasite transmission. Nat. Commun. 2018, 9, 3805. [Google Scholar] [CrossRef] [Green Version]
- Miguel-Blanco, C.; Molina, I.; Bardera, A.I.; Díaz, B.; de Las Heras, L.; Lozano, S.; González, C.; Rodrigues, J.; Delves, M.J.; Ruecker, A.; et al. Hundreds of dual-stage antimalarial molecules discovered by a functional gametocyte screen. Nat. Commun. 2017, 8, 15160. [Google Scholar] [CrossRef] [Green Version]
- Delves, M.; Lafuente-Monasterio, M.J.; Upton, L.; Ruecker, A.; Leroy, D.; Gamo, F.-J.; Sinden, R. Fueling Open Innovation for Malaria Transmission-Blocking Drugs: Hundreds of Molecules Targeting Early Parasite Mosquito Stages. Front. Microbiol. 2019, 10, 2134. [Google Scholar] [CrossRef]
- Azzaz, H.H.; Murad, H.A.; Morsy, T.A. Utility of ionophores for ruminant animals: A review. Asian J. Anim. Sci. 2015, 9, 254–265. [Google Scholar]
- D’Alessandro, S.; Corbett, Y.; Ilboudo, D.P.; Misiano, P.; Dahiya, N.; Abay, S.M.; Habluetzel, A.; Grande, R.; Gismondo, M.R.; Dechering, K.J.; et al. Salinomycin and Other Ionophores as a New Class of Antimalarial Drugs with Transmission-Blocking Activity. Antimicrob. Agents Chemother. 2015, 59, 5135–5144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutkowski, J.; Brzezinski, B. Structures and Properties of Naturally Occurring Polyether Antibiotics. BioMed Res. Int. 2013, 2013, 1–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleck, W.F.; Strauss, D.G.; Meyer, J.; Porstendorfer, G. Fermentation, isolation, and biological activity of maduramycin: A new antibiotic from Actinomadura rubra. Z. Allg. Mikrobiol. 1978, 18, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.I.; Magle, C.T.; Czesny, B.; Turturice, B.A.; Huang, R.; Zheng, W.; Vaidya, A.B.; Williamson, K.C. Maduramicin Rapidly Eliminates Malaria Parasites and Potentiates the Gametocytocidal Activity of the Pyrazoleamide PA21A050. Antimicrob. Agents Chemother. 2016, 60, 1492–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Tanaka, T.Q.; Magle, C.T.; Huang, W.; Southall, N.; Huang, R.; Dehdashti, S.J.; McKew, J.C.; Williamson, K.C.; Zheng, W. Chemical signatures and new drug targets for gametocytocidal drug development. Sci. Rep. 2014, 4, 3743. [Google Scholar] [CrossRef] [PubMed]
- Bolscher, J.M.; Koolen, K.M.J.; Van Gemert, G.J.; Van De Vegte-Bolmer, M.G.; Bousema, T.; Leroy, D.; Sauerwein, R.W.; Dechering, K.J. A combination of new screening assays for prioritization of transmission-blocking antimalarials reveals distinct dynamics of marketed and experimental drugs. J. Antimicrob. Chemother. 2015, 70, 1357–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelièvre, J.; Almela, M.J.; Lozano, S.; Miguel, C.; Franco, V.; Leroy, D.; Herreros, E. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence “transmission blocking” assay. PLoS ONE 2012, 7, e35019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almela, M.J.; Lozano, S.; Lelievre, J.; Colmenarejo, G.; Coterón, J.M.; Rodrigues, J.; González, C.; Herreros, E. A New Set of Chemical Starting Points with Plasmodium falciparum Transmission-Blocking Potential for Antimalarial Drug Discovery. PLoS ONE 2015, 10, e0135139. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, S.; Silvestrini, F.; Dechering, K.; Corbett, Y.; Parapini, S.; Timmerman, M.; Galastri, L.; Basilico, N.; Sauerwein, R.; Alano, P.; et al. A Plasmodium falciparum screening assay for anti-gametocyte drugs based on parasite lactate dehydrogenase detection. J. Antimicrob. Chemother. 2013, 68, 2048–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alessandro, S.; Camarda, G.; Corbett, Y.; Siciliano, G.; Parapini, S.; Cevenini, L.; Michelini, E.; Roda, A.; Leroy, D.; Taramelli, D. A chemical susceptibility profile of the Plasmodium falciparum transmission stages by complementary cell-based gametocyte assays. J. Antimicrob. Chemother. 2016, 71, 1148–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czesny, B.; Goshu, S.; Cook, J.L.; Williamson, K.C. The Proteasome Inhibitor Epoxomicin Has Potent Plasmodium falciparum Gametocytocidal Activity. Antimicrob. Agents Chemother. 2009, 53, 4080–4085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminake, M.N.; Arndt, H.D.; Pradel, G. The proteasome of malaria parasites: A multi-stage drug target for chemotherapeutic intervention? Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaMonte, G.M.; Almaliti, J.; Bibo-Verdugo, B.; Keller, L.; Zou, B.Y.; Yang, J.; Antonova-Koch, Y.; Orjuela-Sanchez, P.; Boyle, C.A.; Vigil, E.; et al. Development of a Potent Inhibitor of the Plasmodium Proteasome with Reduced Mammalian Toxicity. J. Med. Chem. 2017, 60, 6721–6732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminake, M.N.; Schoof, S.; Sologub, L.; Leubner, M.; Kirschner, M.; Arndt, H.D.; Pradel, G. Thiostrepton and derivatives exhibit antimalarial and gametocytocidal activity by dually targeting parasite proteasome and apicoplast. Antimicrob. Agents Chemother. 2011, 55, 1338–1348. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, R.; Markovic, M.; Machado, M.; Franke-Fayard, B.; Mendes, A.M.; Prudêncio, M. Bioluminescence Method for In Vitro Screening of Plasmodium Transmission-Blocking Compounds. Antimicrob. Agents Chemother. 2017, 61, 02699-16. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, R.G.; Huitt-Roehl, C.R.; Tripathi, A.K.; Cheng, Y.-Q.; Bosch, J.; Townsend, C.A.; Dimopoulos, G. Chromobacterium spp. mediate their anti-Plasmodium activity through secretion of the histone deacetylase inhibitor romidepsin. Sci. Rep. 2018, 8, 6176. [Google Scholar] [CrossRef]
- Delves, M.J.; Ramakrishnan, C.; Blagborough, A.M.; Leroy, D.; Wells, T.N.; Sinden, R.E. A high-throughput assay for the identification of malarial transmission-blocking drugs and vaccines. Int. J. Parasitol. 2012, 42, 999–1006. [Google Scholar] [CrossRef]
- Held, J.; Gebru, T.; Kalesse, M.; Jansen, R.; Gerth, K.; Müller, R.; Mordmüller, B. Antimalarial activity of the myxobacterial macrolide chlorotonil A. Antimicrob. Agents Chemother. 2014, 58, 6378–6384. [Google Scholar] [CrossRef] [Green Version]
- Vos, M.W.; Stone, W.J.R.; Koolen, K.M.; Van Gemert, G.-J.; Van Schaijk, B.; Leroy, D.; Sauerwein, R.W.; Bousema, T.; Dechering, K.J. A semi-automated luminescence based standard membrane feeding assay identifies novel small molecules that inhibit transmission of malaria parasites by mosquitoes. Sci. Rep. 2015, 5, 18704. [Google Scholar] [CrossRef]
- Pastrana-Mena, R.; Mathias, D.K.; Delves, M.; Rajaram, K.; King, J.G.; Yee, R.; Trucchi, B.; Verotta, L.; Dinglasan, R.R. A Malaria Transmission-Blocking (+)-Usnic Acid Derivative PreventsPlasmodiumZygote-to-Ookinete Maturation in the Mosquito Midgut. ACS Chem. Biol. 2016, 11, 3461–3472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Niu, G.; Franca, C.M.; Dong, Y.; Wang, X.; Butler, N.S.; Dimopoulos, G.; Li, J. Anopheles Midgut FREP1 Mediates Plasmodium Invasion. J. Boil. Chem. 2015, 290, 16490–16501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, G.; Wang, B.; Zhang, G.; King, J.B.; Cichewicz, R.H.; Li, J. Targeting mosquito FREP1 with a fungal metabolite blocks malaria transmission. Sci. Rep. 2015, 5, 14694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucknall, R.A.; Moores, H.; Simms, R.; Hesp, B. Antiviral Effects of Aphidicolin, a New Antibiotic Produced by Cephalosporium aphidicola. Antimicrob. Agents Chemother. 1973, 4, 294–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janse, C.J.; Van Der Klooster, P.F.; Van Der Kaay, H.J.; Van Der Ploeg, M.; Overdulve, J.P. DNA synthesis in Plasmodium berghei during asexual and sexual development. Mol. Biochem. Parasitol. 1986, 20, 173–182. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Peatey, C.L.; Leroy, D.; Gardiner, D.L.; Trenholme, K.R. Anti-malarial drugs: How effective are they against Plasmodium falciparum gametocytes? Malar. J. 2012, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Delves, M.; Plouffe, D.; Scheurer, C.; Meister, S.; Wittlin, S.; Winzeler, E.A.; Sinden, R.E.; Leroy, D. The Activities of Current Antimalarial Drugs on the Life Cycle Stages of Plasmodium: A Comparative Study with Human and Rodent Parasites. PLoS Med. 2012, 9, e1001169. [Google Scholar] [CrossRef] [Green Version]
- Bousema, J.T.; Schneider, P.; Gouagna, L.C.; Drakeley, C.; Tostmann, A.; Houben, R.; Githure, J.I.; Ord, R.; Sutherland, C.J.; Omar, S.A.; et al. Moderate Effect of Artemisinin-Based Combination Therapy on Transmission of Plasmodium falciparum. J. Infect. Dis. 2006, 193, 1151–1159. [Google Scholar] [CrossRef] [Green Version]
- Price, R.N.; Nosten, F.; Luxemburger, C.; Ter Kuile, F.O.; Paiphun, L.; Chongsuphajaisiddhi, T.; White, N. Effects of artemisinin derivatives on malaria transmissibility. Lancet 1996, 347, 1654–1658. [Google Scholar] [CrossRef]
- White, N.J.; Ashley, E.A.; Recht, J.; Delves, M.; Ruecker, A.; Smithuis, F.; Eziefula, A.C.; Bousema, T.; Drakeley, C.; Chotivanich, K.; et al. Assessment of therapeutic responses to gametocytocidal drugs in Plasmodium falciparum malaria. Malar. J. 2014, 13, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaich, J.N.; Mathias, D.K.; Torto, B.; Jackson, B.T.; Tao, D.; Ebrahimi, B.; Tarimo, B.B.; Cheseto, X.; Foster, W.A.; Dinglasan, R.R. The Nonartemisinin Sesquiterpene Lactones Parthenin and Parthenolide Block Plasmodium falciparum Sexual Stage Transmission. Antimicrob. Agents Chemother. 2016, 60, 2108–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyo, P.; Kunyane, P.; Selepe, M.A.; Eloff, J.N.; Niemand, J.; Louw, A.I.; Maharaj, V.J.; Birkholtz, L.-M. Bioassay-guided isolation and identification of gametocytocidal compounds from Artemisia afra (Asteraceae). Malar. J. 2019, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Abay, S.M.; Lucantoni, L.; Dahiya, N.; Dori, G.U.; Dembo, E.G.; Esposito, F.; Lupidi, G.; Ogboi, J.S.; Ouédraogo, R.K.; Sinisi, A.; et al. Plasmodium transmission blocking activities of Vernonia amygdalina extracts and isolated compounds. Malar. J. 2015, 14, 288. [Google Scholar] [CrossRef] [Green Version]
- Sirignano, C.; Snene, A.; Rigano, D.; Tapanelli, S.; Formisano, C.; Luciano, P.; El Mokni, R.; Hammami, S.; Tenoh, A.R.; Habluetzel, A.; et al. Angeloylated Germacranolides from Daucus virgatus and Their Plasmodium Transmission Blocking Activity. J. Nat. Prod. 2017. [Google Scholar] [CrossRef]
- Sirignano, C.; Snene, A.; Tenoh, A.R.; El Mokni, R.; Rigano, D.; Habluetzel, A.; Hammami, S.; Taglialatela-Scafati, O. Daucovirgolides I-L, four congeners of the antimalarial daucovirgolide G from Daucus virgatus. Fitoterapia 2019, 137, 104188. [Google Scholar] [CrossRef]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Kumar, N.; Aikawa, M.; Grotendorst, C. Plasmodium gallinaceum: Critical role for microtubules in the transformation of zygotes into ookinetes. Exp. Parasitol. 1985, 59, 239–247. [Google Scholar] [CrossRef]
- Jones, I.W.; Denholm, A.A.; Ley, S.V.; Lovell, H.; Wood, A.; Sinden, R.E. Sexual development of malaria parasites is inhibited in vitro by the neem extract azadirachtin, and its semi-synthetic analogues. FEMS Microbiol. Lett. 1994, 120, 267–273. [Google Scholar] [CrossRef]
- Lucantoni, L.; Yerbanga, S.R.; Lupidi, G.; Pasqualini, L.; Esposito, F.; Habluetzel, A. Transmission blocking activity of a standardized neem (Azadirachta indica) seed extract on the rodent malaria parasite Plasmodium berghei in its vector Anopheles stephensi. Malar. J. 2010, 9, 66. [Google Scholar] [CrossRef]
- Yerbanga, S.R.; Lucantoni, L.; Ouédraogo, R.K.; Da, D.F.; Yao, F.A.; Yameogo, B.; Churcher, T.S.; Lupidi, G.; Taglialatela-Scafati, O.; Gouagna, L.C.; et al. Transmission blocking activity of Azadirachta indica and Guiera senegalensis extracts on the sporogonic development of Plasmodium falciparum field isolates in Anopheles coluzzii mosquitoes. Parasites Vectors 2014, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Dhar, R.; Zhang, K.; Talwar, G.P.; Garg, S.; Kumar, N. Inhibition of the growth and development of asexual and sexual stages of drug-sensitive and resistant strains of the human malaria parasite Plasmodium falciparum by Neem (Azadirachta indica) fractions. J. Ethnopharmacol. 1998, 61, 31–39. [Google Scholar] [CrossRef]
- Udeinya, J.I.; Shu, E.; Quakyi, I.; Ajayi, F. An Antimalarial Neem Leaf Extract has Both Schizonticidal and Gametocytocidal Activities. Am. J. Ther. 2008, 15, 108–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udeinya, I.J.; Brown, N.; Shu, E.N.; Udeinya, F.I.; Quakeyie, I. Fractions of an antimalarial neem-leaf extract have activities superior to chloroquine, and are gametocytocidal. Ann. Trop. Med. Parasitol. 2006, 100, 17–22. [Google Scholar] [CrossRef]
- Dahiya, N.; Chianese, G.; Abay, S.M.; Taglialatela-Scafati, O.; Esposito, F.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Christophides, G.K.; Habluetzel, A.; et al. In vitro and ex vivo activity of an Azadirachta indica A.Juss. seed kernel extract on early sporogonic development of Plasmodium in comparison with azadirachtin A, its most abundant constituent. Phytomedicine 2016, 23, 1743–1752. [Google Scholar] [CrossRef] [Green Version]
- Tapanelli, S.; Chianese, G.; Lucantoni, L.; Yerbanga, R.S.; Habluetzel, A.; Taglialatela-Scafati, O. Transmission blocking effects of neem (Azadirachta indica) seed kernel limonoids on Plasmodium berghei early sporogonic development. Fitoterapia 2016, 114, 122–126. [Google Scholar] [CrossRef]
- Billker, O.; Shaw, M.K.; Jones, I.W.; Ley, S.V.; Mordue, A.J.; Sinden, R.E. Azadirachtin Disrupts Formation of Organised Microtubule Arrays during Microgametogenesis of Plasmodium berghei. J. Eukaryot. Microbiol. 2002, 49, 489–497. [Google Scholar] [CrossRef]
- Bousema, T.; Okell, L.; Shekalaghe, S.; Griffin, J.; Omar, S.; Sawa, P.; Sutherland, C.; Sauerwein, R.; Ghani, A.C.; Drakeley, C. Revisiting the circulation time of Plasmodium falciparum gametocytes: Molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar. J. 2010, 9, 136. [Google Scholar] [CrossRef] [Green Version]
- Mackerras, M.; Ercole, Q. Observations on the action of quinine, atebrin and plasmoquine on the gametocytes of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 1949, 42, 455–463. [Google Scholar] [CrossRef]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef] [Green Version]
- Goodman, C.D.; Austarheim, I.; Mollard, V.; Mikolo, B.; Malterud, K.E.; McFadden, G.I.; Wangensteen, H. Natural products from Zanthoxylum heitzii with potent activity against the malaria parasite. Malar. J. 2016, 15, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onambele, L.A.; Riepl, H.; Fischer, R.; Pradel, G.; Prokop, A.; Aminake, M.N. Synthesis and evaluation of the antiplasmodial activity of tryptanthrin derivatives. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forkuo, A.D.; Ansah, C.; Gyan, B.; Mancama, D.; Theron, A. In vitro antimalarial interaction and transmission-blocking activity of cryptolepine. Planta Medica 2016, 81, S1–S381. [Google Scholar]
- Wright, C.W.; Addae-Kyereme, J.; Breen, A.G.; Brown, J.E.; Cox, M.F.; Croft, S.L.; Gökçek, Y.; Kendrick, H.; Phillips, R.M.; Pollet, P.L. Synthesis and evaluation of cryptolepine analogues for their potential as new antimalarial agents. J. Med. Chem. 2001, 44, 3187–3194. [Google Scholar] [CrossRef]
- Arango, E.M.; Londoño, B.; Segura, C.; Solarte, Y.; Herrera, S.; Saez, J.; Blair, S.; Carmona-Fonseca, J.; Londono-Renteria, B.L. Prevention of sporogony ofPlasmodium vivax inAnopheles albimanus by steroids ofSolanum nudum Dunal (Solanaceae). Phytotherapy Res. 2006, 20, 444–447. [Google Scholar] [CrossRef]
- Krieg, R.; Jortzik, E.; Goetz, A.A.; Blandin, S.; Wittlin, S.; Elhabiri, M.; Rahbari, M.; Nuryyeva, S.; Voigt, K.; Dahse, H.M. Arylmethylamino steroids as antiparasitic agents. Nat. Comm. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Soré, H.; D’Alessandro, S.; Sanon, S.; Parapini, S.; Tiono, A.; Valea, I.; Sirima, S.; Hilou, A.; Taramelli, D. In vitro inhibitory activity against plasmodium falciparum sexual and asexual stages of medicinal plants used in burkina. Int. J. Curr. Med. Pharm. Res. 2017, 4, 2976–2983. [Google Scholar]
- Lopatriello, A.; Soré, H.; Habluetzel, A.; Parapini, S.; D’Alessandro, S.; Taramelli, D.; Taglialatela-Scafati, O. Identification of a potent and selective gametocytocidal antimalarial agent from the stem barks of Lophira lanceolata. Bioorganic Chem. 2019, 93, 103321. [Google Scholar] [CrossRef]
- Soré, H.; Lopatriello, A.; Ebstie, Y.A.; Guedoung, A.R.T.; Hilou, A.; Pereira, J.A.; Kijjoa, A.; Habluetzel, A.; Taglialatela-Scafati, O. Plasmodium stage-selective antimalarials from Lophira lanceolata stem bark. Phytochemistry 2020, 174, 112336. [Google Scholar] [CrossRef]
- Paton, D.G.; Childs, L.M.; Itoe, M.A.; Holmdahl, I.E.; Buckee, C.O.; Catteruccia, F. Exposing Anopheles mosquitoes to antimalarials blocks Plasmodium parasite transmission. Nature 2019, 567, 239. [Google Scholar] [CrossRef]
- Amoah, L.E.; Kakaney, C.; Kwansa-Bentum, B.; Asamoah Kusi, K. Activity of Herbal Medicines on Plasmodium falciparum Gametocytes: Implications for Malaria Transmission in Ghana. PLoS ONE 2015, 10, e0142587. [Google Scholar] [CrossRef] [PubMed]
- Buckling, A.; Crooks, L.; Read, A. Plasmodium chabaudi: Effect of antimalarial drugs on gametocytogenesis. Exp. Parasitol. 1999, 93, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio, L.; Gonzalez, I.; Olliaro, P.; Taylor, W.R. Artemisinin-based combination therapy for uncomplicated Plasmodium falciparum malaria in Colombia. Malar. J. 2007, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peatey, C.L.; Skinner-Adams, T.S.; Dixon, M.W.; McCarthy, J.S.; Gardiner, D.L.; Trenholme, K.R. Effect of antimalarial drugs on Plasmodium falciparum gametocytes. J. Infect. Dis. 2009, 200, 1518–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowunmi, A.; Fateye, B. Plasmodium falciparum gametocytaemia in Nigerian children: Before, during and after treatment with antimalarial drugs. Trop. Med. Int. Health 2003, 8, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Rongnoparut, P.; Duangkaew, P.; Prasopthum, A.; Pouyfung, P. Structure-Function Relationships of Phytochemicals in Control of Mosquito Vectors. Curr. Org. Chem. 2016, 20, 2649–2673. [Google Scholar] [CrossRef] [Green Version]
- Kishore, N.; Mishra, B.B.; Tiwari, V.K.; Tripathi, V.; Lall, N. Natural products as leads to potential mosquitocides. Phytochem. Rev. 2014, 13, 587–627. [Google Scholar] [CrossRef] [Green Version]
- Chaccour, C.; Rabinovich, N.R. Ivermectin to reduce malaria transmission II. Considerations regarding clinical development pathway. Malar. J. 2017, 16, 166. [Google Scholar] [CrossRef] [Green Version]
- Õmura, S.; Crump, A. The life and times of ivermectin—A success story. Nat. Rev. Microbiol. 2004, 2, 984–989. [Google Scholar] [CrossRef]
- Chaccour, C.J.; Kobylinski, K.C.; Bassat, Q.; Bousema, T.; Drakeley, C.; Alonso, P.; Foy, B.D. Ivermectin to reduce malaria transmission: A research agenda for a promising new tool for elimination. Malar. J. 2013, 12, 153. [Google Scholar] [CrossRef] [Green Version]
- Campbell, W.; Fisher, M.; Stapley, E.; Albers-Schonberg, G.; Jacob, T. Ivermectin: A potent new antiparasitic agent. Science 1983, 221, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Yates, D.M.; Wolstenholme, A.J. An ivermectin-sensitive glutamate-gated chloride channel subunit from Dirofilaria immitis. Int. J. Parasitol. 2004, 34, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, A.L.; Bastiaens, G.J.; Tiono, A.B.; Guelbéogo, W.M.; Kobylinski, K.C.; Ouédraogo, A.; Barry, A.; Bougouma, E.C.; Nebie, I.; San Ouattara, M. Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: A double-blind, randomized, clinical trial. Clin. Infect. Dis. 2015, 60, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Shoop, W.; Egerton, J.; Eary, C.; Haines, H.; Michael, B.; Mrozik, H.; Eskola, P.; Fisher, M.; Slayton, L.; Ostlind, D. Eprinomectin: A novel avermectin for use as a topical endectocide for cattle. Int. J. Parasitol. 1996, 26, 1237–1242. [Google Scholar] [CrossRef]
- Poché, R.M.; Burruss, D.; Polyakova, L.; Poché, D.M.; Garlapati, R.B. Treatment of livestock with systemic insecticides for control of Anopheles arabiensis in western Kenya. Malar. J. 2015, 14, 351. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, L.P.; Sandri, T.L.; de Melo, E.J.T.; Fendel, R.; Kremsner, P.G.; Mordmüller, B.; Held, J. Ivermectin impairs the development of sexual and asexual stages of Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 2019, 63, e00085-19. [Google Scholar] [CrossRef] [Green Version]
- Wachira, S.W.; Omar, S.; Jacob, J.W.; Wahome, M.; Alborn, H.T.; Spring, D.R.; Masiga, D.K.; Torto, B. Toxicity of six plant extracts and two pyridone alkaloids from Ricinus communis against the malaria vector Anopheles gambiae. Parasites Vectors 2014, 7, 312. [Google Scholar] [CrossRef] [Green Version]
- Hien, D.F.D.S.; Dabiré, K.R.; Roche, B.; Diabaté, A.; Yerbanga, R.S.; Cohuet, A.; Yameogo, B.K.; Gouagna, L.-C.; Hopkins, R.J.; Ouedraogo, G.A. Plant-mediated effects on mosquito capacity to transmit human malaria. PLoS Pathog. 2016, 12, e1005773. [Google Scholar] [CrossRef] [Green Version]
- Dembo, E.G.; Abay, S.M.; Dahiya, N.; Ogboi, J.S.; Christophides, G.K.; Lupidi, G.; Chianese, G.; Lucantoni, L.; Habluetzel, A. Impact of repeated NeemAzal®-treated blood meals on the fitness of Anopheles stephensi mosquitoes. Parasites Vectors 2015, 8, 1–13. [Google Scholar] [CrossRef]
- Overgaard, H.J.; Sirisopa, P.; Mikolo, B.; Malterud, K.E.; Wangensteen, H.; Zou, Y.-F.; Paulsen, B.S.; Massamba, D.; Duchon, S.; Corbel, V. Insecticidal activities of bark, leaf and seed extracts of Zanthoxylum heitzii against the African malaria vector Anopheles gambiae. Molecules 2014, 19, 21276–21290. [Google Scholar] [CrossRef] [Green Version]
- Rottmann, M.; McNamara, C.; Yeung, B.K.; Lee, M.C.; Zou, B.; Russell, B.; Seitz, P.; Plouffe, D.M.; Dharia, N.V.; Tan, J. Spiroindolones, a new and potent chemotype for the treatment of malaria. Science 2010, 329, 1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charman, S.A.; Arbe-Barnes, S.; Bathurst, I.C.; Brun, R.; Campbell, M.; Charman, W.N.; Chiu, F.C.; Chollet, J.; Craft, J.C.; Creek, D.J. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc. Natl. Acad. Sci. USA 2011, 108, 4400–4405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Pelt-Koops, J.C.; Pett, H.E.; Graumans, W.; Van Der Vegte-Bolmer, M.; Van Gemert, G.J.; Rottmann, M.; Yeung, B.K.; Diagana, T.T.; Sauerwein, R.W. The Spiroindolone Drug Candidate NITD609 Potently Inhibits Gametocytogenesis and Blocks Plasmodium falciparum Transmission to Anopheles Mosquito Vector. Antimicrob. Agents Chemother. 2012, 56, 3544–3548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, N.J.; Pukrittayakamee, S.; Phyo, A.P.; Rueangweerayut, R.; Nosten, F.; Jittamala, P.; Jeeyapant, A.; Jain, J.P.; Lefèvre, G.; Li, R. Spiroindolone KAE609 for falciparum and vivax malaria. N. Engl. J. Med. 2014, 371, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Lughadha, E.N.; Govaerts, R.; Belyaeva, I.; Black, N.; Lindon, H.; Allkin, R.; Magill, R.E.; Nicolson, N. Counting counts: Revised estimates of numbers of accepted species of flowering plants, seed plants, vascular plants and land plants with a review of other recent estimates. Phytotaxa 2016, 272, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Meshnick, S.R.; Dobson, M.J. The history of antimalarial drugs. In Antimalarial Chemotherapy; Springer: Berlin/Heidelberg, Germany, 2001; pp. 15–25. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Drugs and drug candidates from marine sources: An assessment of the current “state of play”. Planta Med. 2016, 82, 775–789. [Google Scholar] [CrossRef] [Green Version]
- Reader, J.; van der Watt, M.E.; Taylor, D.; Le Manach, C.; Mittal, N.; Ottilie, S.; Theron, A.; Moyo, P.; Erlank, E.; Nardini, L. Multistage and transmission-blocking targeted antimalarials discovered from the open-source MMV Pandemic Response Box. bioRxiv 2020. [Google Scholar] [CrossRef]
- Abraham, M.; Gagaring, K.; Martino, M.L.; Vanaerschot, M.; Plouffe, D.M.; Calla, J.; Godinez-Macias, K.P.; Du, A.Y.; Wree, M.; Antonova-Koch, Y. Probing the Open Global Health Chemical Diversity Library for Multistage-Active Starting Points for Next-Generation Antimalarials. ACS Infect. Dis. 2020, 6, 613–628. [Google Scholar] [CrossRef]
- Spangenberg, T.; Burrows, J.N.; Kowalczyk, P.; McDonald, S.; Wells, T.N.; Willis, P. The open access malaria box: A drug discovery catalyst for neglected diseases. PLoS ONE 2013, 8, e62906. [Google Scholar] [CrossRef] [Green Version]
- Bapela, M.J.; Heyman, H.; Senejoux, F.; Meyer, J.M. 1H NMR-based metabolomics of antimalarial plant species traditionally used by Vha-Venda people in Limpopo Province, South Africa and isolation of antiplasmodial compounds. J. Ethnopharmacol. 2019, 228, 148–155. [Google Scholar] [CrossRef]
- Heyman, H.M.; Senejoux, F.; Seibert, I.; Klimkait, T.; Maharaj, V.J.; Meyer, J.J.M. Identification of anti-HIV active dicaffeoylquinic-and tricaffeoylquinic acids in Helichrysum populifolium by NMR-based metabolomic guided fractionation. Fitoterapia 2015, 103, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, J.J.; Todd, D.A.; Egan, J.M.; Raja, H.A.; Oberlies, N.H.; Kvalheim, O.M.; Cech, N.B. Biochemometrics for natural products research: Comparison of data analysis approaches and application to identification of bioactive compounds. J. Nat. Prod. 2016, 79, 376–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, V.; Potterat, O.; Louvel Sv Hamy Fo Mojarrab, M.; Sanglier, J.-J.; Klimkait, T.; Hamburger, M. Library-based discovery and characterization of daphnane diterpenes as potent and selective HIV inhibitors in Daphne gnidium. J. Nat. Prod. 2011, 75, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, C.C.; Britt, J.R.; Evans, J.R.; Akee, R.K.; Whitt, J.A.; Trinh, S.K.; Harris, M.J.; Thompson, J.R.; Ewing, T.L.; Shipley, S.M. NCI program for natural product discovery: A publicly-accessible library of natural product fractions for high-throughput screening. ACS Chem. Biol. 2018, 13, 2484–2497. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyo, P.; Mugumbate, G.; Eloff, J.N.; Louw, A.I.; Maharaj, V.J.; Birkholtz, L.-M. Natural Products: A Potential Source of Malaria Transmission Blocking Drugs? Pharmaceuticals 2020, 13, 251. https://doi.org/10.3390/ph13090251
Moyo P, Mugumbate G, Eloff JN, Louw AI, Maharaj VJ, Birkholtz L-M. Natural Products: A Potential Source of Malaria Transmission Blocking Drugs? Pharmaceuticals. 2020; 13(9):251. https://doi.org/10.3390/ph13090251
Chicago/Turabian StyleMoyo, Phanankosi, Grace Mugumbate, Jacobus N. Eloff, Abraham I. Louw, Vinesh J. Maharaj, and Lyn-Marié Birkholtz. 2020. "Natural Products: A Potential Source of Malaria Transmission Blocking Drugs?" Pharmaceuticals 13, no. 9: 251. https://doi.org/10.3390/ph13090251
APA StyleMoyo, P., Mugumbate, G., Eloff, J. N., Louw, A. I., Maharaj, V. J., & Birkholtz, L. -M. (2020). Natural Products: A Potential Source of Malaria Transmission Blocking Drugs? Pharmaceuticals, 13(9), 251. https://doi.org/10.3390/ph13090251