Synthesis and In Vitro Assessment of pH-Sensitive Human Serum Albumin Conjugates of Pirarubicin
Abstract
:1. Introduction
2. Results
2.1. Synthesis of THP-EMCH
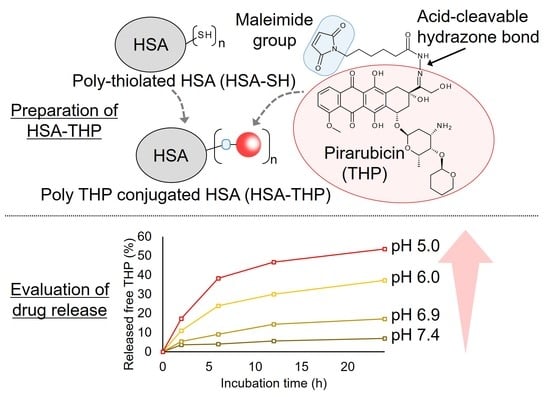
2.2. Synthesis of HSA-THP2 and HSA-THP4
2.3. Release of Free THP from HSA Conjugates under Acidic pH Conditions
2.4. In Vitro Cytotoxicity of HSA-THP2 and HSA-THP4
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Maleimide Hydrazone Derivatives of THP (THP-EMCH)
4.3. Synthesis of Poly-Thiolated HSA (HSA-SH)
4.4. Synthesis of HSA Conjugates of THP (HSA-THP)
4.5. In Vitro Drug Release from HSA-THPs
4.6. 1H-NMR, 13C-NMR, ESI-Mass, Dynamic Light Scattering (DLS), Zeta Potential, Fluorescence Spectroscopy, and HPLC
4.7. In Vitro Cytotoxicity Assay
4.8. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Umezawa, H.; Takahashi, Y.; Kinoshita, M.; Naganawa, H.; Masuda, T.; Ishizuka, M.; Tatsuta, K.; Takeuchi, T. Tetrahydropyranyl derivatives of daunomycin and adriamycin. J. Antibiot. 1979, 32, 1082–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunimoto, S.; Miura, K.; Takahashi, Y.; Takeuchi, T.; Umezawa, H. Rapid uptake by cultured tumor cells and intracellular behavior of 4′-O-tetrahydropyranyladriamycin. J. Antibiot. 1983, 36, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, S.; Miura, K.; Umezawa, K.; Xu, C.Z.; Masuda, T.; Takeuchi, T.; Umezawa, H. Cellular uptake and efflux and cytostatic activity of 4′-O-tetrahydropyranyladriamycin in adriamycin-sensitive and resistant tumor cell lines. J. Antibiot. 1984, 37, 1697–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuruo, T.; Iida, H.; Tsukagoshi, S.; Sakurai, Y. 4′-O-tetrahydropyranyladriamycin as a potential new antitumor agent. Cancer Res. 1982, 42, 1462–1467. [Google Scholar] [PubMed]
- Koh, E.; Ueda, Y.; Nakamura, T.; Kobayashi, A.; Katsuta, S.; Takahashi, H. Apoptosis in young rats with adriamycin-induced cardiomyopathy―Comparison with pirarubicin, a new anthracycline derivative. Pediatr. Res. 2002, 51, 256–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Maeda, H.; Matsumura, Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit. Rev. Ther. Drug Carr. Syst. 1989, 6, 193–210. [Google Scholar]
- Maeda, H.; Miyamoto, Y.; Seymour, L.W.; Seymour, L.W. Conjugates of anticancer agents and polymers: Advantages of macromolecular therapeutics in vivo. Bioconjug. Chem. 1992, 3, 351–362. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.K.; Jain, R.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [Green Version]
- Malugin, A.; Kopecková, P.; Kopecek, J. Liberation of doxorubicin from HPMA copolymer conjugate is essential for the induction of cell cycle arrest and nuclear fragmentation in ovarian carcinoma cells. J. Control. Release 2007, 124, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, H.; Akita, H.; Harashima, H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: A strategy for overcoming the PEG dilemma. Adv. Drug Deliv. Rev. 2011, 63, 152–160. [Google Scholar] [CrossRef]
- Tsukigawa, K.; Nakamura, H.; Fang, J.; Otagiri, M.; Maeda, H. Effect of different chemical bonds in pegylation of zinc protoporphyrin that affects drug release, intracellular uptake, and therapeutic effect in the tumor. Eur. J. Pharm. Biopharm. 2015, 89, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Etrych, T.; Tsukigawa, K.; Nakamura, H.; Chytil, P.; Fang, J.; Ulbrich, K.; Otagiri, M.; Maeda, H. Comparison of the pharmacological and biological properties of HPMA copolymer-pirarubicin conjugates: A single-chain copolymer conjugate and its biodegradable tandem-diblock copolymer conjugate. Eur. J. Pharm. Sci. 2017, 106, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Koziolová, E.; Chytil, P.; Tsukigawa, K.; Fang, J.; Haratake, M.; Ulbrich, K.; Etrych, T.; Maeda, H. Pronounced Cellular uptake of pirarubicin versus that of other anthracyclines: Comparison of HPMA copolymer conjugates of pirarubicin and doxorubicin. Mol. Pharm. 2016, 13, 4106–4115. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; Rotin, D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar] [PubMed]
- Gerweck, L.E.; Seetharaman, K. Cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996, 56, 1194–1198. [Google Scholar] [PubMed]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Schnitzer, J.E.; Oh, P. Antibodies to SPARC inhibit albumin binding to SPARC, gp60, and microvascular endothelium. Am. J. Physiol. 1992, 263, H1872–H1879. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Gao, Y.; Wei, N.; Zhao, X.; Wang, C.; Li, Y.; Xiu, X.; Cui, J. Direct comparison of two albumin-based paclitaxel-loaded nanoparticle formulations: Is the crosslinked version more advantageous? Int. J. Pharm. 2014, 468, 15–25. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z. Albumin carriers for cancer theranostics: A conventional platform with new promise. Adv. Mater. 2016, 28, 10557–10566. [Google Scholar] [CrossRef] [PubMed]
- An, F.F.; Zhang, X.H. Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef] [PubMed]
- Katayama, N.; Nakajou, K.; Komori, H.; Uchida, K.; Yokoe, J.; Yasui, N.; Yamamoto, H.; Kai, T.; Sato, M.; Nakagawa, T.; et al. Design and evaluation of S-nitrosylated human serum albumin as a novel anticancer drug. J. Pharmacol. Exp. Ther. 2008, 325, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishima, Y.; Chen, D.; Fang, J.; Maeda, H.; Minomo, A.; Kragh-Hansen, U.; Kai, T.; Maruyama, T.; Otagiri, M. S-Nitrosated human serum albumin dimer is not only a novel anti-tumor drug but also a potentiator for anti-tumor drugs with augmented EPR effects. Bioconjug. Chem. 2012, 23, 264–271. [Google Scholar] [CrossRef]
- Ishima, Y.; Fang, J.; Kragh-Hansen, U.; Yin, H.; Liao, L.; Katayama, N.; Watanabe, H.; Kai, T.; Suenaga, A.; Maeda, H.; et al. Tuning of poly-S-nitrosated human serum albumin as superior antitumor nanomedicine. J. Pharm. Sci. 2014, 103, 2184–2188. [Google Scholar] [CrossRef]
- Marks, D.S.; Vita, J.A.; Folts, J.D.; Keaney, J.F.; Welch, G.N.; Loscalzo, J. Inhibition of neointimal proliferation in rabbits after vascular injury by a single treatment with a protein adduct of nitric oxide. J. Clin. Investig. 1995, 96, 2630–2638. [Google Scholar] [CrossRef] [Green Version]
- Ewing, J.F.; Young, D.V.; Janero, D.R.; Garvey, D.S.; Grinnell, T.A. Nitrosylated bovine serum albumin derivatives as pharmacologically active nitric oxide congeners. J. Pharmacol. Exp. Ther. 1997, 283, 947–954. [Google Scholar]
- Walker, G.F.; Fella, C.; Pelisek, J.; Fahrmeir, J.; Boeckle, S.; Ogris, M.; Wagner, E. Toward synthetic viruses: Endosomal pH-triggered deshielding of targeted polyplexes greatly enhances gene transfer in vitro and in vivo. Mol. Ther. 2005, 11, 418–425. [Google Scholar] [CrossRef]
- Nakamura, H.; Etrych, T.; Chytil, P.; Ohkubo, M.; Fang, J.; Ulbrich, K.; Maeda, H. Two step mechanisms of tumor selective delivery of N-(2-hydroxypropyl)methacrylamide copolymer conjugated with pirarubicin via an acid-cleavable linkage. J. Control. Release 2014, 174, 81–87. [Google Scholar] [CrossRef]
- Mo, G.; Hu, X.; Liu, S.; Yue, J.; Wang, R.; Huang, Y.; Jing, X. Influence of coupling bonds on the anti-tumor activity of polymer-pirarubicin conjugates. Eur. J. Pharm. Sci. 2012, 46, 329–335. [Google Scholar] [CrossRef]
- Pu, Y.; Chang, S.; Yuan, H.; Wang, G.; He, B.; Gu, Z. The anti-tumor efficiency of poly(l-glutamic acid) dendrimers with polyhedral oligomeric silsesquioxane cores. Biomaterials 2013, 34, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Zhao, M.; Wu, L.; Luo, K.; Pu, Y.; He, B. Tumor-pH-Sensitive PLLA-Based Microsphere with Acid Cleavable Acetal Bonds on the Backbone for Efficient Localized Chemotherapy. Biomacromolecules 2018, 19, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cai, H.; Zhang, H.; Zhu, H.; Gu, Z.; Gong, Q.; Luo, K. Stimuli-responsive polymer-doxorubicin conjugate: Antitumor mechanism and potential as nano-prodrug. Acta Biomater. 2019, 84, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Tsukigawa, K.; Liao, L.; Nakamura, H.; Fang, J.; Greish, K.; Otagiri, M.; Maeda, H. Synthesis and therapeutic effect of styrene-maleic acid copolymer-conjugated pirarubicin. Cancer Sci. 2015, 106, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.F. Removal of fatty acids from serum albumin by charcoal treatment. J. Biol. Chem. 1967, 242, 173–181. [Google Scholar] [PubMed]








| IC50 (µg/mL THP Equivalent) | Free THP | HSA-THP2 | HSA-THP4 |
|---|---|---|---|
| pH 7.4 | 0.11 ± 0.01 | 1.01 ± 0.09 | 1.01 ± 0.12 |
| pH 6.9 | 0.15 ± 0.01 | 0.65 ± 0.06 * | 0.63 ± 0.06 * |
| pH 6.5 | 0.22 ± 0.02 | 0.55 ± 0.04 * | 0.54 ± 0.07 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsukigawa, K.; Imoto, S.; Yamasaki, K.; Nishi, K.; Tsutsumi, T.; Yokoyama, S.; Ishima, Y.; Otagiri, M. Synthesis and In Vitro Assessment of pH-Sensitive Human Serum Albumin Conjugates of Pirarubicin. Pharmaceuticals 2021, 14, 22. https://doi.org/10.3390/ph14010022
Tsukigawa K, Imoto S, Yamasaki K, Nishi K, Tsutsumi T, Yokoyama S, Ishima Y, Otagiri M. Synthesis and In Vitro Assessment of pH-Sensitive Human Serum Albumin Conjugates of Pirarubicin. Pharmaceuticals. 2021; 14(1):22. https://doi.org/10.3390/ph14010022
Chicago/Turabian StyleTsukigawa, Kenji, Shuhei Imoto, Keishi Yamasaki, Koji Nishi, Toshihiko Tsutsumi, Shoko Yokoyama, Yu Ishima, and Masaki Otagiri. 2021. "Synthesis and In Vitro Assessment of pH-Sensitive Human Serum Albumin Conjugates of Pirarubicin" Pharmaceuticals 14, no. 1: 22. https://doi.org/10.3390/ph14010022
APA StyleTsukigawa, K., Imoto, S., Yamasaki, K., Nishi, K., Tsutsumi, T., Yokoyama, S., Ishima, Y., & Otagiri, M. (2021). Synthesis and In Vitro Assessment of pH-Sensitive Human Serum Albumin Conjugates of Pirarubicin. Pharmaceuticals, 14(1), 22. https://doi.org/10.3390/ph14010022