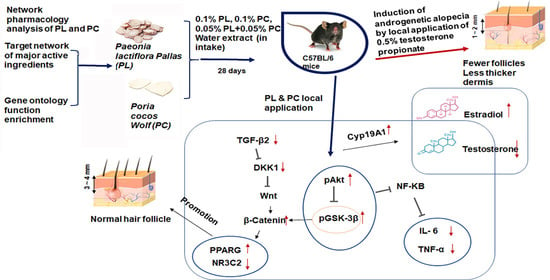
Alleviation of Androgenetic Alopecia with Aqueous Paeonia lactiflora and Poria cocos Extract Intake through Suppressing the Steroid Hormone and Inflammatory Pathway
Abstract
:1. Introduction
2. Results
2.1. Screening of Active Ingredients in PL and PC and Predictions of Potential Targets for AGA Treatment Using TCMSP
2.2. Network Topology Diagram of “Active Component-Disease-Target” for PL and PC
2.3. PPI Network Analysis and GO Enrichment Analysis
2.4. Contents of Index Compounds in PL and PC, Food and Herbal Intake, Body Weight, and Fat Weight in Mice
2.5. Hair Growth Status and Clinical Hair Growth Scores
2.6. Histopathology of Dorsal Skins
2.7. Serum Testosterone, 17β-Estradiol, and Triglyceride Concentrations
2.8. mRNA Expressions of AGA-Related Genes in Dorsal Skin
2.9. Phosphorylation of Akt and GSK-3β in the Dorsal Skin
3. Discussion
4. Materials and methods
4.1. Screening PL and PC Active Ingredients as Potential Medications
4.2. A Network Topology Map of PL and PC Bioactive Ingredient Targets and AGA Targets
4.3. Protein–Protein Interaction (PPI) Network Construction and Gene Ontology (GO) Enrichment Analysis
4.4. Preparation of PL and PC Water Extracts and Analysis of Their Index Compounds
4.5. Experimental Animal Model
4.6. Experimental Design
4.7. Assess the Degree of Hair Growth in AGA Mice
4.8. Blood and Tissue Collection and Serum Analysis
4.9. Histopathological Analysis
4.10. Relative mRNA Expressions of pro-Inflammatory Cytokines, Nuclear Receptors, and Wnt Signaling-Related Genes in Skin Tissues
4.11. Western Blot
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nanes, B.A. Androgenetic alopecia in COVID-19: Compared to what? J. Am. Acad. Dermatol. 2020, 83, e451. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Y.; Liu, K.; Lu, Y.; Hu, Y.; Zhou, X. Therapeutic effect of Impatiens balsamina, Lawsonia inermis L. and Henna on androgenetic alopecia in mice. J. South. Med Univ. 2019, 39, 1376–1380. [Google Scholar] [CrossRef]
- Dhariwala, M.Y.; Ravikumar, P. An overview of herbal alternatives in androgenetic alopecia. J. Cosmet. Dermatol. 2019, 18, 966–975. [Google Scholar] [CrossRef]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Sadgrove, N.J. The ‘Bald’ Phenotype (Androgenetic Alopecia) is Caused by the High Glycaemic, High Cholesterol and Low Mineral ‘Western Diet’. Trends Food Sci. Technol. 2021, 116, 1170–1178. [Google Scholar] [CrossRef]
- Lee, S.W.; Juhasz, M.; Mobasher, P.; Ekelem, C.; Mesinkovska, N.A. A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. J. Drugs Dermatol. 2018, 17, 457–463. [Google Scholar]
- Mysore, V.; Shashikumar, B.M. Guidelines on the use of finasteride in androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 128–134. [Google Scholar] [CrossRef]
- Almohanna, H.M.; Perper, M.; Tosti, A. Safety concerns when using novel medications to treat alopecia. Expert Opin. Drug Saf. 2018, 17, 1115–1128. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Iamsumang, W.; Leerunyakul, K. Topical finasteride for the treatment of male androgenetic alopecia and female pattern hair loss: A review of the current literature. J. Dermatol. Treat. 2020, 1–6. [Google Scholar] [CrossRef]
- Piraccini, B.M.; Blume-Peytavi, U.; Scarci, F.; Jansat, J.M.; Falqués, M.; Otero, R.; Tamarit, M.L.; Galván, J.; Tebbs, V.; Massana, E. Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: A phase III, randomized, controlled clinical trial. J. Eur. Acad. Dermatol. Venereol. JEADV 2021. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Tampucci, S.; Burgalassi, S.; Chetoni, P.; Lenzi, C.; Pirone, A.; Mailland, F. Topical formulations containing finasteride. Part I: In vitro permeation/penetration study and in vivo pharmacokinetics in hairless rat. J. Pharm. Sci. 2014, 103, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Noubarani, M.; Rostamkhani, H.; Erfan, M.; Kamalinejad, M.; Eskandari, M.R.; Babaeian, M.; Salamzadeh, J. Effect of Adiantum Capillus veneris Linn on an Animal Model of Testosterone-Induced Hair Loss. Iran. J. Pharm. Res. IJPR 2014, 13, 113–118. [Google Scholar] [PubMed]
- Sadgrove, N.J. The new paradigm for androgenetic alopecia and plant-based folk remedies: 5α-reductase inhibition, reversal of secondary microinflammation and improving insulin resistance. J. Ethnopharmacol. 2018, 227, 206–236. [Google Scholar] [CrossRef]
- Mao, X.; Chen, W.J.; Li, Y.F.; Li, W.J.; Li, T.X.; Wang, X.Y.; Guo, M.Q.; Zhang, Y.Q.; Lin, N. An exploration into the therapeutic effects and molecular mechanisms of paeoniflorin in the treatment of adjuvant-induced arthritis rats by a network pharmacology-based research strategy. Acta Pharm. 2019, 54, 2000–2010. [Google Scholar]
- Parker, S.; May, B.; Zhang, C.; Zhang, A.L.; Lu, C.; Xue, C.C. A Pharmacological Review of Bioactive Constituents of Paeonia lactiflora Pallas and Paeonia veitchii Lynch. Phytother Res. 2016, 30, 1445–1473. [Google Scholar] [CrossRef]
- He, D.Y.; Dai, S.M. Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall., a traditional chinese herbal medicine. Front. Pharmacol. 2011, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jin, D.Z.; Xiao, L.; Zhu, X.Z. Paeoniflorin attenuates chronic cerebral hypoperfusion-induced learning dysfunction and brain damage in rats. Brain Res. 2006, 1089, 162–170. [Google Scholar] [CrossRef]
- Jia, X.; Ma, L.; Li, P.; Chen, M.; He, C. Prospects of Poria cocos polysaccharides: Isolation process, structural features and bioactivities. Trends Food Sci. Technol. 2016, 54, 52–62. [Google Scholar]
- Feng, Y.L.; Lei, P.; Tian, T.; Yin, L.; Chen, D.Q.; Chen, H.; Mei, Q.; Zhao, Y.Y.; Lin, R.C. Diuretic activity of some fractions of the epidermis of Poria cocos. J. Ethnopharmacol. 2013, 150, 1114–1118. [Google Scholar] [CrossRef]
- Sun, S.S.; Wang, K.; Ma, K.; Bao, L.; Liu, H.W. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 2019, 17, 3–14. [Google Scholar] [CrossRef]
- Ríos, J.L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Goren, A.; Cadegiani, F.A.; Wambier, C.G.; Vano-Galvan, S.; Tosti, A.; Shapiro, J.; Mesinkovska, N.A.; Ramos, P.M.; Sinclair, R.; Lupi, O.; et al. Androgenetic alopecia may be associated with weaker COVID-19 T-cell immune response: An insight into a potential COVID-19 vaccine booster. Med. Hypotheses 2021, 146, 110439. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Na, J.; Bak, D.H.; Lee, B.C.; Lee, E.; Choi, M.J.; Ryu, C.H.; Lee, S.; Mun, S.K.; Park, B.C.; et al. Development of finasteride polymer microspheres for systemic application in androgenic alopecia. Int. J. Mol. Med. 2019, 43, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-D.; Feng, Y.; Ma, L.-Y.; Li, X.; Ding, W.-F.; Chen, X.-M. Hair growth promoting effect of white wax and policosanol from white wax on the mouse model of testosterone-induced hair loss. Biomed. Pharmacother. 2017, 89, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Orăsan, M.S.; Coneac, A. Evaluation of Animal Models Suitable for Hair Research and Regeneration, Experimental Animal Models of Human Diseases—An Effective Therapeutic Strategy; IntechOpen: London, UK, 2017. [Google Scholar]
- Lee, S.Y.; Lee, D.J.; Kwon, K.; Lee, C.H.; Shin, H.J.; Kim, J.E.; Ha, K.T.; Jeong, H.S.; Seo, H.S. Cornu cervi pantotrichum Pharmacopuncture Solution Facilitate Hair Growth in C57BL/6 Mice. J. Pharmacopunct. 2016, 19, 122–128. [Google Scholar] [CrossRef]
- Gang, L.; Bo, X.; Liang, X.-Z.; Gai, S.-S.; Xia, C.-M.; Yan, B.-Z.; Li, J.-C. Study on the molecular mechanism of osteoporosis treated by Epimedium based on network pharmacology. Chin. Pharmacol. Bull. 2018, 34, 267–273. [Google Scholar]
- Fu, D.; Huang, J.; Li, K.; Chen, Y.; He, Y.; Sun, Y.; Guo, Y.; Du, L.; Qu, Q.; Miao, Y.; et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed Pharm. 2021, 137, 111247. [Google Scholar] [CrossRef]
- English, R.S., Jr. A hypothetical pathogenesis model for androgenic alopecia: Clarifying the dihydrotestosterone paradox and rate-limiting recovery factors. Med. Hypotheses 2018, 111, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Bak, M.J.; Lee, C.; Jun, M.; Jeong, W.S. Hair Regenerative Mechanisms of Red Ginseng Oil and Its Major Components in the Testosterone-Induced Delay of Anagen Entry in C57BL/6 Mice. Molecules 2017, 22, 1505. [Google Scholar] [CrossRef] [Green Version]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef]
- Sun, Y. Biological activities and potential health benefits of polysaccharides from Poria cocos and their derivatives. Int. J. Biol. Macromol. 2014, 68, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wei, W.; Zhang, L.; Xu, H.-M. Effects and mechanisms of total glucosides of paeony on synoviocytes activities in rat collagen-induced arthritis. J. Ethnopharmacol. 2009, 121, 43–48. [Google Scholar] [CrossRef]
- Ong, M.; Cheng, J.; Jin, X.; Lao, W.; Johnson, M.; Tan, Y.; Qu, X. Paeoniflorin extract reverses dexamethasone-induced testosterone over-secretion through downregulation of cytochrome P450 17A1 expression in primary murine theca cells. J. Ethnopharmacol. 2019, 229, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, B.; Zhang, N. Network target for screening synergistic drug combinations with application to traditional Chinese medicine. BMC Syst. Biol. 2011, 5, S10. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Su, W. Recent progress in applying network pharmacology to research of Chinese materia medica. Chin. Tradit. Herb. Drugs 2016, 47, 2938–2942. [Google Scholar]
- Ansari, S.; Bari, A.; Ullah, R.; Mathanmohun, M.; Veeraraghavan, V.P.; Sun, Z. Gold nanoparticles synthesized with Smilax glabra rhizome modulates the anti-obesity parameters in high-fat diet and streptozotocin induced obese diabetes rat model. J. Photochem. Photobiol. B Biol. 2019, 201, 111643. [Google Scholar] [CrossRef]
- Zhao, J.; Niu, X.; Yu, J.; Xiao, X.; Li, W.; Zang, L.; Hu, Z.; Siu-Po Ip, P.; Li, W. Poria cocos polysaccharides attenuated ox-LDL-induced inflammation and oxidative stress via ERK activated Nrf2/HO-1 signaling pathway and inhibited foam cell formation in VSMCs. Int. Immunopharmacol. 2020, 80, 106173. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Jing, J.; Wu, X.; Lv, Z. Serum Levels of Androgen-Associated Hormones Are Correlated with Curative Effect in Androgenic Alopecia in Young Men. Med. Sci. Monit. 2018, 24, 7770–7777. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, B.; Li, Y.; Han, L.; Tang, X.; Deng, W.; Lai, W.; Wan, M. Dihydrotestosterone Regulates Hair Growth Through the Wnt/β-Catenin Pathway in C57BL/6 Mice and In Vitro Organ Culture. Front. Pharmacol. 2020, 10, 1528. [Google Scholar] [CrossRef] [Green Version]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. The Inflammatory Aspect of Male and Female Pattern Hair Loss. J. Inflamm. Res. 2020, 13, 879–881. [Google Scholar] [CrossRef]
- Liu, J.; Yu, J.; Peng, X. Poria cocos Polysaccharides Alleviates Chronic Nonbacterial Prostatitis by Preventing Oxidative Stress, Regulating Hormone Production, Modifying Gut Microbiota, and Remodeling the DNA Methylome. J. Agric. Food Chem. 2020, 68, 12661–12670. [Google Scholar] [CrossRef]
- Wang, Q.S.; Gao, T.; Cui, Y.L.; Gao, L.N.; Jiang, H.L. Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm. Biol. 2014, 52, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Andy, G.; John, M.; Mirna, S.; Rachita, D.; Michael, K.; Maja, K.; Aseem, S.; Zeljana, B. Controversies in the treatment of androgenetic alopecia: The history of finasteride. Dermatol. Ther. 2019, 32, e12647. [Google Scholar] [CrossRef]
- Duborija-Kovacevic, N.; Jakovljevic, V.; Sabo, A.; Tomic, Z. Anti-nociceptive and anti-inflammatory properties of 5alpha-reductase inhibitor finasteride in experimental animals. Eur. J. Drug Metab. Pharmacokinet. 2008, 33, 181–186. [Google Scholar] [CrossRef]
- Available online: http://tcmspw.com/tcmsp.php (accessed on 4 March 2020).
- Available online: Https://www.genecards.org/ (accessed on 31 March 2020).
- Available online: Https://omim.org/ (accessed on 6 May 2020).
- Available online: Https://www.malacards.org/ (accessed on 20 May 2020).
- Available online: Https://string-db.org/ (accessed on 4 June 2020).
- Available online: Http://www.bioconductor.org/ (accessed on 24 June 2020).
- Zhang, B.; Zhang, R.W.; Yin, X.Q.; Lao, Z.Z.; Zhang, Z.; Wu, Q.G.; Yu, L.W.; Lai, X.P.; Wan, Y.H.; Li, G. Inhibitory activities of some traditional Chinese herbs against testosterone 5α-reductase and effects of Cacumen platycladi on hair re-growth in testosterone-treated mice. J. Ethnopharmacol. 2016, 177, 1–9. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Im, Y.N.; Youm, J.Y.; Lee, H.K.; Im, S.Y. l-Glutamine Attenuates DSS-Induced Colitis via Induction of MAPK Phosphatase-1. Nutrients 2018, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ellis, J.A.; Sinclair, R.; Harrap, S.B. Androgenetic alopecia: Pathogenesis and potential for therapy. Expert Rev. Mol. Med. 2002, 4, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.S.; Ko, B.-S.; Ryuk, J.A.; Park, S. Tetragonia tetragonioides Protected against Memory Dysfunction by Elevating Hippocampal Amyloid-β Deposition through Potentiating Insulin Signaling and Altering Gut Microbiome Composition. Int. J. Mol. Sci. 2020, 21, 2900. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| AGA-Con (N = 12) | AGA-Positive (N = 12) | AGA-PL (N = 12) | AGA- PC (N = 12) | AGA-MIX (N = 11) | Normal-Con (N = 11) | |
|---|---|---|---|---|---|---|
| Final weight (g) | 25.6 ± 0.76 | 26.8 ± 0.55 | 26.7 ± 0.58 | 25.4 ± 0.69 | 25.5 ± 0.39 | 26.5 ± 0.38 |
| Weight gain (g/8 weeks) | 3.71 ± 0.32 ab | 4.13 ± 0.38 a | 3.83 ± 0.39 ab | 2.64 ± 0.38 b | 2.55 ± 0.42 b | 3.25 ± 0.31 ab |
| Food intake (g/day) | 4.10 ± 0.31 | 3.59 ± 0.20 | 4.08 ± 0.33 | 3.48 ± 0.40 | 3.78 ± 0.27 | 3.22 ± 0.28 |
| PL or PC intake (mg/kg bw/day) | - | - | 153 ± 12 | 139 ± 16 | 148 ± 11 | - |
| Efficiency of food | 0.97 ± 0.10 ab | 1.14 ± 0.07 a | 0.94 ± 0.08 ab | 0.76 ± 0.13 ab | 0.67 ± 0.09 b | 1.09 ± 0.12 a |
| Epididymal fat (g) | 0.53 ± 0.06 b | 0.68 ± 0.06 a | 0.55 ± 0.07 b | 0.49 ± 0.04 b | 0.40 ± 0.03 c | 0.59 ± 0.04 b |
| Retroperitoneal fat (g) | 0.18 ± 0.02 | 0.25 ± 0.02 | 0.20 ± 0.04 | 0.19 ± 0.02 | 0.15 ± 0.02 | 0.22 ± 0.02 |
| Total visceral fat (g) | 0.72 ± 0.07 b | 0.93 ± 0.09 a | 0.75 ± 0.11 b | 0.67 ± 0.06 b | 0.55 ± 0.04 c | 0.81 ± 0.05 b |
| AGA-Con (N = 12) | AGA-Positive (N = 12) | AGA-PL (N = 12) | AGA- PC (N = 12) | AGA-MIX (N = 11) | Normal-Con (N = 11) | |
|---|---|---|---|---|---|---|
| Serum testosterone (ng/mL) | 27.10 ± 0.81 a | 27.83 ± 0.62 a | 21.15 ± 0.78 b | 23.88 ± 0.38 ab | 24.54 ± 0.56 ab | 21.23 ± 1.33 b |
| Serum 17β-estradiol (ng/mL) | 13.95 ± 2.01 b | 16.14 ± 1.86 ab | 19.38 ± 0.86 a | 17.69 ± 1.84 ab | 16.78 ± 1.39 ab | 18.71 ± 1.86 a |
| Serum total cholesterol (mg/dL) | 149.39 ± 6.71 | 149.96 ± 8.72 | 137.10 ± 5.04 | 135.53 ± 2.99 | 137.28 ± 4.35 | 144.98 ± 4.20 |
| Serum triglyceride (mg/dL) | 63.68 ± 4.96 a | 64.19 ± 4.15 a | 47.51 ± 3.48 ab | 34.64 ± 2.86 b | 58.06 ± 2.80 a | 46.65 ± 2.67 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Cao, S.; Yuan, H.; Park, S. Alleviation of Androgenetic Alopecia with Aqueous Paeonia lactiflora and Poria cocos Extract Intake through Suppressing the Steroid Hormone and Inflammatory Pathway. Pharmaceuticals 2021, 14, 1128. https://doi.org/10.3390/ph14111128
Zhang T, Cao S, Yuan H, Park S. Alleviation of Androgenetic Alopecia with Aqueous Paeonia lactiflora and Poria cocos Extract Intake through Suppressing the Steroid Hormone and Inflammatory Pathway. Pharmaceuticals. 2021; 14(11):1128. https://doi.org/10.3390/ph14111128
Chicago/Turabian StyleZhang, Ting, Shihua Cao, Heng Yuan, and Sunmin Park. 2021. "Alleviation of Androgenetic Alopecia with Aqueous Paeonia lactiflora and Poria cocos Extract Intake through Suppressing the Steroid Hormone and Inflammatory Pathway" Pharmaceuticals 14, no. 11: 1128. https://doi.org/10.3390/ph14111128
APA StyleZhang, T., Cao, S., Yuan, H., & Park, S. (2021). Alleviation of Androgenetic Alopecia with Aqueous Paeonia lactiflora and Poria cocos Extract Intake through Suppressing the Steroid Hormone and Inflammatory Pathway. Pharmaceuticals, 14(11), 1128. https://doi.org/10.3390/ph14111128